Urea Kinetics: Impact of Urea Rebound and Residual Renal Function on the spKt/V and eKt/V Relationship
Urea kinetic modeling (UKM) and calculation of a urea Kt/V is the most widely accepted method to quantify the dose of dialysis. UKM also provides a measure of the protein catabolic rate (PCR), which is equivalent to dietary protein intake in the metabolically stable patient. The calculated Kt/V is proportional to the pre-post dialysis blood urea nitrogen (BUN) decrease. The single pool Kt/Vurea (spKt/V) is calculated from the pre-dialysis BUN and the post-dialysis BUN and also takes into consideration the volume of ultrafiltration and urea generation during dialysis. One caveat is that spKt/V tends to overestimate the amount of urea removal by up to 25%. It does not account for the presence of the post-dialysis rebound in urea that occurs between 30 and 60 minutes after the dialysis session. To address this issue, the concept of the equilibrated Kt/V (eKt/V), which applies double pool kinetics, was developed.
Impact of Urea Rebound on spKt/V, eKt/V and PCRn
At the end of the dialysis session, the concentration of BUN is lower in the blood and extracellular fluid than in cells. This is due to sequestration of urea in the tissues. Following dialysis, urea diffuses out of the tissues back into the blood. The difference between the two BUNs is the “rebound” (see figure 1). The spKt/V calculated using the end of dialysis BUN will be higher than the eKt/V, which is calculated from the BUN after post-dialysis equilibration. It is the eKt/V that more accurately reflects the effective dose of dialysis. The rebound phenomenon and effects on Kt/V and the normalized PCR (PCRn) are illustrated in Fig 1. The BUN at the end of dialysis can be used to calculate the equilibrated BUN value using validated equations or by keeping the patient for 30-60 min to take another blood sample.
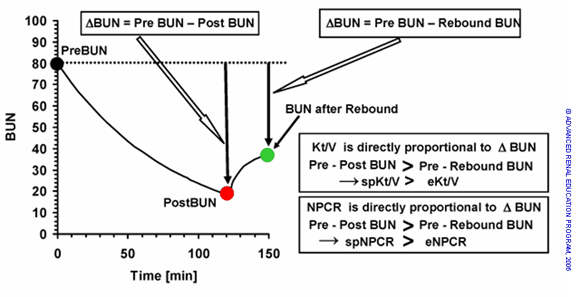 Figure 1. The Kt/V and PCRn are both calculated from the magnitude of BUN decrease during dialysis. The apparent drop in BUN will always be larger before rebound occurs. Since spKt/V and spnPCR are calculated from Pre BUN minus Post BUN, while eKt/V and ePCRn are calculated from Pre BUN minus Rebound BUN, spKt/V and spPCRn will always be larger than eKt/V and ePCRn.
Figure 1. The Kt/V and PCRn are both calculated from the magnitude of BUN decrease during dialysis. The apparent drop in BUN will always be larger before rebound occurs. Since spKt/V and spnPCR are calculated from Pre BUN minus Post BUN, while eKt/V and ePCRn are calculated from Pre BUN minus Rebound BUN, spKt/V and spPCRn will always be larger than eKt/V and ePCRn.
The magnitude of the rebound is determined almost entirely by the rate at which the dialysis dose is delivered. The “rate of dialysis” is defined by the spKt/V divided by the treatment time (t), which equals K/V (K= the delivered clearance and V= the volume of distribution of urea equivalent to the total body water). The greater the K/V the greater is the rebound. Since most patients are treated at about the same blood flows (300 to 400 mL/min) and with the same dialyzer urea clearance (K), the smallest patients (with small Vs and shorter times) will tend to have the highest ratios of K/V and the highest rebounds. spKt/V can be used to determine adequate dialysis dosing targets, but concerns may arise regarding patients who may rebound more than others and receive less adequate dialysis. A popular approach for accounting for the effect of post-dialysis urea rebound on dialysis dose has been to estimate the eKt/V as a linear function of the spKt/V and the rate of dialysis (K/V).
Various equations can be used for predicting post-dialysis urea rebound including the Daugirdas-Schneditz and Tattersal equations. Relationships between spKt/V and eKt/V are illustrated in Fig 2A, where the ratio (eKt/V)/(spKt/V) is plotted as a function of treatment time t. The Tattersall equation was used to calculate eKt/V over an spKt/V range of 1.3 to 1.7 and independent of volume, dialyzer clearance and urea generation (Gu), with treatment times fixed at six levels ranging from 2.0 to 4.5 hrs. The Tattersall equation is shown below for arterial access and venous access.
Arterial access: eKt⁄V= spKt⁄V x (t/(t+35))
Venous access: eKt⁄V= spKt⁄V x (t/(t+22))
Over the ranges calculated, eKt/V is a highly linear function of spKt/V when time is held constant, and a family of six lines is seen in Fig 2A. In Fig 2A, the eKt/V dose target of 1.2 is depicted as a horizontal line on the y axis and the spKt/V dose target of 1.4 as a vertical line on the x axis. The regression lines for different dialysis times are depicted as solid lines for all segments with eKt/V ≥ 1.20 and dashed lines for all segments where eKt/V < 1.20. Note that the regression lines for spKt/V > 1.4 pass through the zone of eKt/V of less than 1.2 because of their high rebound with shorter dialysis times.
In Fig 2B, the required spKt/V to achieve an eKt/V of 1.20 is shown for each treatment time by a vertical arrow to the x axis at the point that each regression line reaches the eKt/V of 1.20. It can be seen that the spKt/V necessary to provide an eKt/V of 1.2 can range from 1.32 to 1.58 over a range of dialysis times. Therefore, an eKt/V can be associated with several spKt/V values, depending on the dialysis time. At steady state, the PCRN is calculated from the amount of urea removed, which is equal to the amount of urea generated (Gu). As noted above, the post-dialysis BUN is artificially low. If it is used for calculating the NPCR, the urea removal will be overestimated since the difference between this BUN concentration and the pre-dialysis BUN of the next treatment are used. The PCRN will therefore behave like the Kt/V.
The Daugirdas-Schneditz rate equations can also be used to predict urea rebound from a non-equilibrated post-dialysis serum urea concentration and the spKt/V (shown below) and depend on access type.
Arterial access: eKt⁄V= spKt⁄V- (0.6 x spKt⁄V)+0.03
Venous access: eKt⁄V= spKt⁄V- (0.47 x spKt⁄V)+0.02
Note that both of the Tattersal and Daugirdas-Schneditz equations have been shown to be accurate for predicting urea rebound in conventional hemodialysis (HD) but not during short HD.
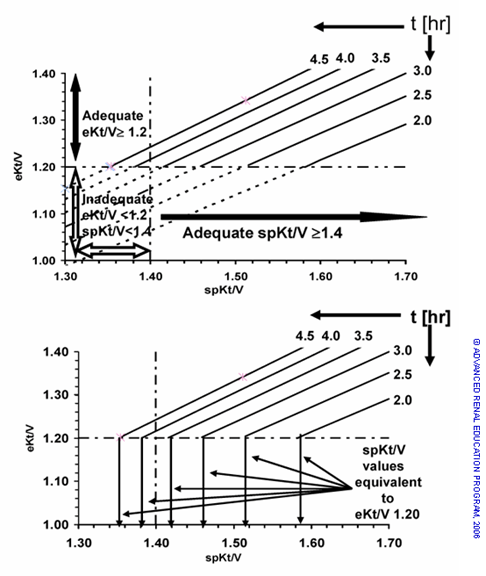 Figure 2. A – Two measurements of the dialysis dose: eKt/V > 1.20 and spKt/V > 1.40. Note that the discrepancy between spKt/V and eKt/V increases as the dialysis time is reduced. B – When t varies in the patient population, there must be a family of spKt/V values ranging from 1.35 – 1.58 to assure all doses equivalent to eKt/V equals 1.20.
Figure 2. A – Two measurements of the dialysis dose: eKt/V > 1.20 and spKt/V > 1.40. Note that the discrepancy between spKt/V and eKt/V increases as the dialysis time is reduced. B – When t varies in the patient population, there must be a family of spKt/V values ranging from 1.35 – 1.58 to assure all doses equivalent to eKt/V equals 1.20.
Impact of residual renal urea clearance (Kru) on eKt/V and ePCRN
Previous studies have shown that a patient’s native residual urea clearance (Kru) can markedly decrease the need for dialysis and have an important influence on mortality. Although the magnitude of this clearance is seemingly small, Kru is a continuous process that serves to attenuate the rise of toxins between dialysis treatments. Several methods outlined in the KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates are available to incorporate Kru into the hemodialyzer clearance.
With respect to thrice weekly hemodialysis, residual renal function can be directly expressed as a quantity of eKt/V (abbreviated as eKrt/V) according to the equation:
eKt⁄V= 4.5 x Kru⁄V
with V in liters and Kru in mL/min; the units of the coefficient 4.5 are L/mL/min.
Assuming a V of 30 L, 1 mL/min Kru contributes an additional 4.5 L of urea clearance per dialysis session, which translates into a gain of eKt/V of 0.15 (4.5 L/30 L = 0.15). If a patient has a Kru of 3 mL/min it will add 3 x 0.15 = 0.45 to the eKt/V provided by hemodialysis (abbreviated as eKdt/V), a highly significant addition. With this Kru being present, the total equilibrated dialysis dose (eKdrt/V) will be simply the sum of eKdt/V and eKrt/V, so that Kru of 3 mL/min brings an eKt/V of 1.2 to 1.65.
The impact of Kru on total eKdrt/V and on the normalized catabolic rate corrected for rebound (ePCRN) is illustrated in Fig 3A and 3B, respectively. In Fig 3A the Kdrt/V is plotted as a function of Kru for an average size patient and four levels of eKdt/V = 0.40, 0.60, 0.80 and 1.00 (these are the y axis values shown when Kru = 0). As discussed above, eKrt/V is additive to eKdt/V. The impact of Kru on PCRN is illustrated in Fig 3B. The UKM NPCR values of 0.6, 0.8, 1.0 and 1.4 are shown with Kru increasing from 0 to 8 mL/min. The “apparent” eNPCR that would be calculated if Kru was present but not measured and assumed to be zero, are depicted. For example, if the NPCR is 1.0 and unmeasured Kru 3 mL/min is present the apparent eNPCR calculated would fall to 0.80 and would be interpreted as marginal protein intake. Thus, residual renal function (when measured and applied) can have a marked impact on eKt/V and eNPCR, exaggerating the differences between the single pool and equilibrated measures.
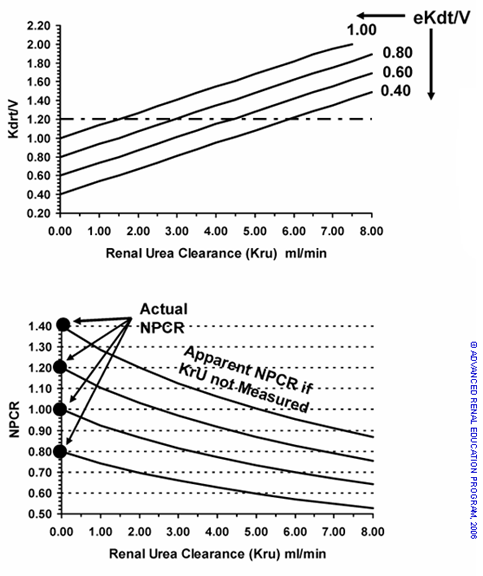 Figure 3. A – Solution for eKdrt/V as a function of Kru for average patient V = 30L. It can be observed that in the average size patient, each ml of Kru increases eKdrt/V by about 0.15 units of eKt/V. B – Solutions for “apparent” NPCR if residual Kru is not included in UKM. The presence of Kru lowers the pre dialysis BUN which will result in spurious lowering of NPCR calculated if Kru is not included in the modeling equations. The error will be ~ 4% for each ml/min of Kru in the average sized patient.
Figure 3. A – Solution for eKdrt/V as a function of Kru for average patient V = 30L. It can be observed that in the average size patient, each ml of Kru increases eKdrt/V by about 0.15 units of eKt/V. B – Solutions for “apparent” NPCR if residual Kru is not included in UKM. The presence of Kru lowers the pre dialysis BUN which will result in spurious lowering of NPCR calculated if Kru is not included in the modeling equations. The error will be ~ 4% for each ml/min of Kru in the average sized patient.
Conclusions:
1. The optimal dialysis dosing target is eKt/V ≥1.20 while the equivalent spKt/V varies over a range depending on treatment time. spKt/V is not equivalent to eKt/V and should not be used as a target representative of an adequate dose. eKt/V is a better measure of clearance than Kt/V.
2. Residual renal function will increase equilibrated Kt/V by about 0.15 units per 1 mL/min of residual renal urea clearance in an average-sized person. The effect of residual renal function on eKt/V can be easily calculated.
3. Residual renal function increasing eKt/V will result in a spurious decrease in apparent protein catabolic rate about -4% per mL/min of clearance when the residual renal function is not measured.
4. Dialysis treatment time and residual renal function (when measured and applied) impact the differences between spKt/V and eKt/V and could affect the achievement of target goals.
Glossary:
- UKM = Urea Kinetic Modeling
- K = Dialyzer clearance
- T = time on dialysis
- V = volume (volume of distribution of urea equivalent to the total body water)
- K/V = rate of dialysis (clearance per Liter of body water per unit time)
- Kt/V = fractional clearance of body water of urea
- Sp = single pool
- E = equilibrated
- eKdt/V = dialysis contribution only to eKt/V
- eKdrt/V = dialysis and renal contribution both to eKt/V
- stdKt/V = Standard Kt/V= the continuous clearance which is therapeutically equivalent to any schedule (number of dialyses per week) and intensity (eKt/V per dialysis) of intermittent dialysis therapy
- PCR = protein catabolic rate Gm/day
- nPCR = normalized catabolic rate, gm/Kg normalized body weight/day
- ePCRn = normalized catabolic rate corrected for rebound
- ru = residual renal function ml/min
- Gu = urea generation mg/min (amount of continuous urea generation in dialysis patients)
References
I. eKt/V Rationale and Urea Rebound (effect on spKt/V)
- Abramson F, Gibson S, Barlee V, Bosch JP. Urea kinetic modeling at high urea clearances: implications for clinical practice. Adv Ren Replace Ther. 1994;1(1):5-14. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7641088.
- Albouze G, Yanai M, Calamai M, Testou D, Jungers P, Man NK. Urea rebound and residual renal function in the calculation of Kt/V and protein catabolic rate. Kidney Int Suppl. 1993;41:S278-S281. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8320937.
- Blake P, Daugirdas J. Quantification And Prescription General Principles. In: Jacobs C, Kjellstrand CM, Koch KM, Winchester JF, eds. Replacement of Renal Function by Dialysis. Springer Netherlands; 1996:619-656.
- Depner T, Beck G, Daugirdas J, Kusek J, Eknoyan G. Lessons from the Hemodialysis (HEMO) Study: An Improved Measure of the Actual Hemodialysis Dose.; 1999. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9915282.
- Evans JHC, Smye SW, Brocklebank JT. Mathematical modelling of haemodialysis in children. Pediatr Nephrol. 1992;6(4):349-353. Available from: https://www.ncbi.nlm.nih.gov/pubmed/1498004.
- Gotch F, Keen M. Kinetic modeling in hemodialysis. In: Nissenson AR, Fine RN, eds. Clinical Dialysis. 4th ed. New York: McGraw-Hill Medical Publication; 2005:153-202.
- Hassaballah AM, Ayadi A, Shaheen MH, Barsoum RS, el-Badry A. Post-dialysis urea rebound. J Egypt Med Assoc. 1974;57(5-6):215-226. Available from: https://www.ncbi.nlm.nih.gov/pubmed/4452764.
- Kerr PG, Argilés A, Canaud B, Flavier JL, Mion CM. Accuracy of Kt/V estimations in high-flux haemodiafiltration using per cent reduction of urea: incorporation of urea rebound. Nephrol Dial Transplant. 1993;8(2):149-153. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8384336.
- Leblanc M, Charbonneau R, Lalumière G, Cartier P, Déziel C. Postdialysis urea rebound: determinants and influence on dialysis delivery in chronic hemodialysis patients. Am J Kidney Dis. 1996;27(2):253-261. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8659502.
- Leypoldt JK, Cheung AK, Deeter RB, Goldfarb-Rumyantzev A, Greene T, Depner TA, Kusek J. Kinetics of urea and β2-microglobulin during and after short hemodialysis treatments. Kidney Int. 2004;66(4):1669-1676. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15458465.
- Marsenić O, Pavlicić D, Bigović G, Peco-Antić A, Jovanović O. Effects of postdialysis urea rebound on the quantification of pediatric hemodialysis. Nephron. 2000;84(2):124-129. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10657712.
- Pedrini LA, Zereik S, Rasmy S. Causes, kinetics and clinical implications of post-hemodialysis urea rebound. Kidney Int. 1988;34(6):817-824. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3210544.
- Spiegel DM, Baker PL, Babcock S, Contiguglia R, Klein M. Hemodialysis urea rebound: the effect of increasing dialysis efficiency. Am J Kidney Dis. 1995;25(1):26-29. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7810527.
- Vanholder R, Burgelman M, De Smet R, Voogeleere P, Ringoir S. Two-pool versus single-pool models in the determination of urea kinetic parameters. Blood Purif. 1996;14(6):437-450. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8915573.
- Yamada T, Akiba T, Marumo F. One-compartment urea kinetic modeling is not acceptable for quantifying the adequacy of hemodialysis: comparison of a one-compartment model with a two-compartment model. Blood Purif. 1996;14(2):128-135. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8785028.
II. Methods to Compute eKt/V
- Bhaskaran S, Tobe S, Saiphoo C, Moldoveanu A, Raj DS, Manuel MA. Blood urea levels 30 minutes before the end of dialysis are equivalent to equilibrated blood urea. ASAIO J. 43(5):M759-M762. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9360148.
- Castro MC, Romão JE, Marcondes M. Measurement of blood urea concentration during haemodialysis is not an accurate method to determine equilibrated post-dialysis urea concentration. Nephrol Dial Transplant. 2001;16(9):1814-1817. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11522863.
- Daugirdas JT, Depner TA, Gotch FA, Greene T, Keshaviah P, Levin NW, Schulman G. Comparison of Methods to Predict Equilibrated Kt/V in the HEMO Pilot Study.; 1997. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9350665.
- Daugirdas JT, Greene T, Depner TA, Leypoldt J, Gotch F, Schulman G, Star R. Factors That Affect Postdialysis Rebound in Serum Urea Concentration, Including the Rate of Dialysis: Results from the HEMO Study.; 2004. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14694173.
- Daugirdas JT. Simplified equations for monitoring Kt/V, PCRn, eKt/V, and ePCRn. Adv Ren Replace Ther. 1995;2(4):295-304. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8591121.
- Fernández EA, Valtuille R, Willshaw P, Perazzo CA. Using artificial intelligence to predict the equilibrated postdialysis blood urea concentration. Blood Purif. 2001;19(3):271-285. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11244187.
- Goldstein SL, Brewer ED. Logarithmic extrapolation of a 15-minute postdialysis BUN to predict equilibrated BUN and calculate double-pool Kt/V in the pediatric hemodialysis population. Am J Kidney Dis. 2000;36(1):98-104. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10873878.
- Goldstein SL, Sorof JM, Brewer ED. Evaluation and prediction of urea rebound and equilibrated Kt/V in the pediatric hemodialysis population. Am J Kidney Dis. 1999;34(1):49-54. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10401015.
- Guh JY, Yang CY, Yang JM, Chen LM, Lai YH. Prediction of equilibrated postdialysis BUN by an artificial neural network in high-efficiency hemodialysis. Am J Kidney Dis. 1998;31(4):638-646. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9531180.
- Gotch F, Keen M. Kinetic modeling in hemodialysis. In: Nissenson AR, Fine RN, eds. Clinical Dialysis. 4th ed. New York: McGraw-Hill Medical Publication; 2005:153-202.
- Jean G, Charra B, Chazot C, Laurent G. Quest for postdialysis urea rebound-equilibrated Kt/V with only intradialytic urea samples. Kidney Int. 1999;56(3):1149-1153. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10469385.
- Kietkajornkul C, Thirakhupt P, Chulamokha Y, Jaiprong S, Kitpanich S. Assessment of the different methods to predict equilibrated Kt/V in pediatric hemodialysis. J Med Assoc Thail = Chotmaihet thangphaet. 2005;88 Suppl 3. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16858957.
- Maduell F, Garcia-Valdecasas J, Garcia H, Hdez-Jaras J, Sigüenza F, Del Pozo C, Giner R, Moll R, Garrigos E. Validation of different methods to calculate Kt/V considering postdialysis rebound. Nephrol Dial Transplant. 1997;12(9):1928-1933. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9306345.
- Marsenić O, Peco-Antić A, Jovanović O. Comparison of two methods for predicting equilibrated Kt/V (eKt/V) using true eKt/V value. Pediatr Nephrol. 1999;13(5):418-422. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10412862.
- Marsenić OD, Pavlicić D, Peco-Antić A, Bigović G, Jovanović O. Prediction of equilibrated urea in children on chronic hemodialysis. ASAIO J. 46(3):283-287. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10826737.
- Ookawara S, Suzuki M, Saitou M, Tabei K. Mathematical analysis of urea rebound in long-term hemodialysis patients. In: Therapeutic Apheresis and Dialysis. ; 2005:167-172. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15828930.
- Pflederer BR, Torrey C, Priester-Coary A, Lau AH, Daugirdas JT. Estimating equilibrated Kt/V from an intradialytic sample: effects of access and cardiopulmonary recirculations. Kidney Int. 1995;48(3):832-837. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7474672.
- Prado M, Roa LM, Palma A, Milán JA. Double target comparison of blood-side methods for measuring the hemodialysis dose. Kidney Int. 2005;68(6):2863-2876. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16316364.
- Smye SW, Dunderdale E, Brownridge G, Will E. Estimation of treatment dose in high-efficiency haemodialysis. Nephron. 1994;67(1):24-29. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8052363.
- Smye SW, Evans JH, Will E, Brocklebank JT. Paediatric haemodialysis: estimation of treatment efficiency in the presence of urea rebound. Clin Phys Physiol Meas. 1992;13(1):51-62. Available from: https://www.ncbi.nlm.nih.gov/pubmed/1563221.
- Smye SW, Tattersall JE, Will EJ. Modeling the postdialysis rebound: the reconciliation of current formulas. ASAIO J. 1992;45(6):562-567. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10593687.
- Tattersall JE, DeTakats D, Chamney P, Greenwood RN, Farrington K. The post-hemodialysis rebound: predicting and quantifying its effect on Kt/V. Kidney Int. 1996;50(6):2094-2102. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8943495.
III. eKt/V and spKt/V For Dialysis Prescriptions
- Daugirdas JT, Greene T, Depner TA, Gotch FA, Star RA. Relationship between apparent (single-pool) and true (double-pool) urea distribution volume. Kidney Int. 1999;56(5):1928-1933. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10571804.
- Elangovan L, Shinaberger CS, Kraut JA, Shinaberger JH. HEMO equilibrated Kt/V goals are difficult to achieve in large male patients. ASAIO J. 47(3):235-239. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11374764.
- Fernández EA, Valtuille R, Presedo JMR, Willshaw P. Comparison of different methods for hemodialysis evaluation by means of ROC curves: from artificial intelligence to current methods. Clin Nephrol. 2005;64(3):205-213. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16175945.
- Goldstein SL, Brem A, Warady BA, Fivush B, Frankenfield D. Comparison of single-pool and equilibrated Kt/V values for pediatric hemodialysis prescription management: Analysis from the Centers for Medicare & Medicaid Services Clinical Performance Measures Project. Pediatr Nephrol. 2006;21(8):1161-1166. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16705459.
- K/DOQI Clinical practice guidelines for hemodialysis adequacy. Am J Kidney Dis. 2006;48 Suppl 1:S2-S90. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16813990.
- Kanagasundaram NS, Greene T, Larive AB, Daugirdas JT, Depner TA, Garcia M, Paganini EP. Prescribing an equilibrated intermittent hemodialysis dose in intensive care unit acute renal failure. Kidney Int. 2003;64(6):2298-2310. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14633155.
- Leypoldt JK, Cheung AK. Revisiting the hemodialysis dose. Semin Dial. 2006;19(2):96-101. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16551284.
- Miwa T, Nakai S, Miwa M, Shinzato T, Segawa K, Maeda K. Which Kt/V is the most valid for assessment of both long mild and short intensive hemodialyses? Nephron. 2002;92(4):827-831. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12399628.
- Tang HL, Tsang WK, Yeung S, Chan HWH, Tong KL. Solute removal index correlates more with equilibrated Kt/V than with single pool Kt/V in haemodialysis patients. Nephrology. 2004;9(1):39-43. Available from: https://www.ncbi.nlm.nih.gov/pubmed/14996308.
IV. eKt/V Associated Outcomes
- Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347(25):2010-2019. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12490682.
- Gotch FA, Levin NW, Port FK, Wolfe RA, Uehlinger DE. Clinical outcome relative to the dose of dialysis is not what you think: the fallacy of the mean. Am J Kidney Dis. 1997;30(1):1-15. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9214395.
- Greene T, Daugirdas J, Depner T, Allon M, Beck G, Chumlea C, Delmez J, Gotch F, Kusek JW, Levin N, et al. Association of achieved dialysis dose with mortality in the hemodialysis study: an example of “dose-targeting bias”. J Am Soc Nephrol. 2005;16(11):3371-3380. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16192421.
- O’Connor AS, Leon JB, Sehgal AR. The relative predictive ability of four different measures of hemodialysis dose. Am J Kidney Dis. 2002;40(6):1289-1294. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12460049.
- Stosovic M, Stanojevic M, Radovic M, Naumovic R, Jovanovic D, Simic S, Marinkovic J, Stankovic S, Djukanovic LJ. Comparative survival analysis of urea kinetic based indices. Int J Artif Organs. 2005;28(6):566-575. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16015566.
- Termorshuizen F, Dekker FW, van Manen JG, Korevaar JC, Boeschoten EW, Krediet RT. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061-1070. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15034110.
- Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35(1):80-88. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10620548.
P/N 102545-01 Rev. A 07/2015
