Transport Status: Classification and Implications
Classifying Peritoneal Membrane Transport Status
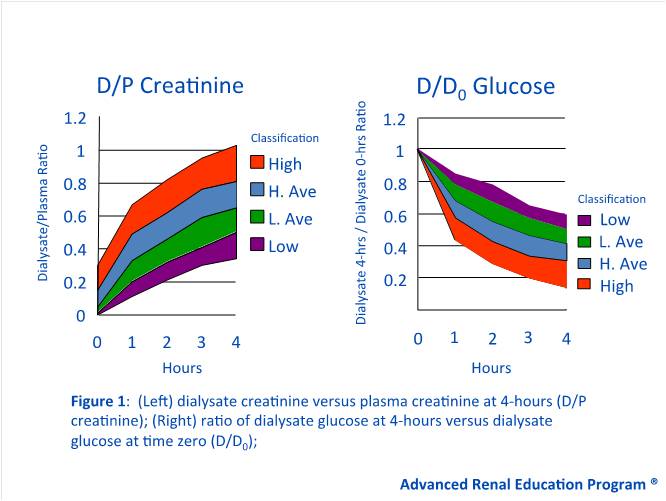
In 1987, Twardowski et al. proposed the first method to classify membrane function by measuring the rate at which solutes equilibrate between the dialysate and body plasma (1). This dialysate-to-plasma ratio, called the D/P ratio, measures the combined effect of diffusion and ultrafiltration (1,2). A low solute D/P means that transport across the peritoneal membrane for a given solute occurs slowly, and equilibrium between the dialysate and plasma is reached gradually. In contrast, a high solute D/P means that transport of a solute across the membrane occurs quickly, and equilibrium is reached sooner (1-3) (Figure 1). D/P ratios are typically assessed for various solutes including urea, creatinine, and sodium.
Another important concept is the D/D0 glucose, which is defined as the dialysate glucose at 4 hours versus the dialysate glucose at time zero (2). Glucose is a common osmotic agent in peritoneal dialysis (PD) solutions, but is limited by absorption from the dialysate into systemic circulation. As absorption occurs, the osmotic gradient dissipates and ultrafiltration is lost. A high D/D0 glucose indicates slow glucose absorption and sustained ultrafiltration, whereas a low D/D0 glucose indicates quick absorption and a rapid loss of ultrafiltration (2) (Figure 1).
Using the D/P ratio of creatinine and D/D0 glucose, patients can be classified into one of four transport categories: High (fast), high average, low average, and low (slow) (1-3) (Figure 1).
Figure 1 – Twardowski Curves: Transport Status Based on the Peritoneal Equilibration Test (PET)
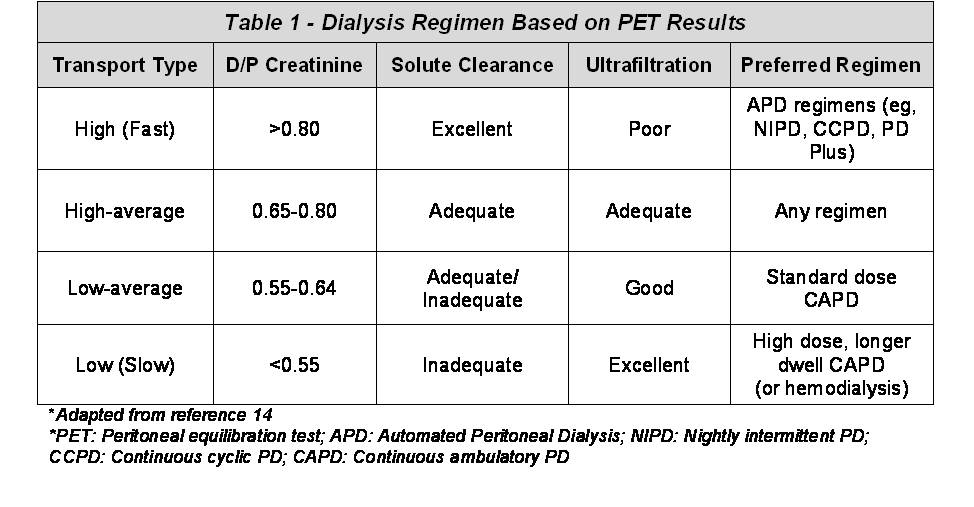
Fast transporters generally have a D/P creatinine greater than 0.80 (1,3). These patients achieve rapid and complete equilibration of small solutes due to a larger functional membrane surface area and higher membrane permeability (1,4,5). However, fast transporters quickly lose their osmotic gradient and achieve poor ultrafiltration because dialysate glucose is rapidly absorbed into the blood (Table 1). Thus, fast transporters have the greatest D/P ratios for creatinine and urea, but the lowest D/D0 glucose.
Unlike fast transporters, slow transporters have the lowest D/P ratios for creatinine and urea, where the D/P creatinine is typically less than 0.55 (1,3). These patients achieve a slower and less complete equilibration for small solutes. On the other hand, slow transporters have the greatest D/D0 glucose due to slower glucose transport across the peritoneal membrane. As a result, they can sustain their osmotic gradient for longer periods and therefore achieve better ultrafiltration (2) (see Table 1). Patients who are high-average or low-average transporters have moderately high or moderately low diffusion and ultrafiltration characteristics (2). Typically, the D/P creatinine for high-average transporters will range from 0.65 to 0.80, while low-average transporters will have a D/P creatinine ranging from 0.55 to 0.64 (3) (See Table 1). However, it is important to remember that such cut-offs may vary based on the geographical area, time on PD, and other factors related to the testing process or population studied (3).
 Other testing methods can be further used to classify patients by: The time required for solutes to achieve 50% equilibration between the dialysate and plasma (called the Pt50) (9); the mass transfer area coefficient (MTAC) of small solutes (10); the amount of proteins lost through large membrane pores (11,12); and a patient’s 24-hour solute clearance (13). More specific information on peritoneal membrane testing methods can be found in the Understanding Testing Methods section of the Peritoneal Dialysis article.
Other testing methods can be further used to classify patients by: The time required for solutes to achieve 50% equilibration between the dialysate and plasma (called the Pt50) (9); the mass transfer area coefficient (MTAC) of small solutes (10); the amount of proteins lost through large membrane pores (11,12); and a patient’s 24-hour solute clearance (13). More specific information on peritoneal membrane testing methods can be found in the Understanding Testing Methods section of the Peritoneal Dialysis article.
Clinical Implications of Peritoneal Transport Status
Using Transport Status to Choose the Best PD Regimen (APD versus CAPD)
Once membrane function has been determined, clinicians can better predict the most appropriate PD regimen for a given patient (3,14,15). As shown in Table 1, slow transporters generally have reduced solute clearance and sustained ultrafiltration, and can therefore be well maintained on modalities allowing for longer dwell times, such as continuous ambulatory peritoneal dialysis (CAPD) (3,14,15). The use of CAPD provides a longer time-period to equilibrate small solutes and allow adequate fluid removal.
In contrast, fast transporters, are in general good candidates for automated PD (APD) regimens that use shorter dwell times. Examples of APD regimens include nightly intermittent peritoneal dialysis (NIPD) or continuous cyclic peritoneal dialysis (CCPD) (3,14-16). The short dwell time allows patients to maximize small solute clearance while still retaining enough dialysate glucose to permit adequate ultrafiltration (5,14-16). Such recommendations for fast transporters have been endorsed by the International Society for Peritoneal Dialysis (ISPD) (17), UK Renal Association (UKRA) (18), and European Best Practice Guidelines (EBPG) (19). However, it should be noted that while small solute clearance is improved with shorter cycles, middle-molecule clearance might be worsened, as these molecules diffuse poorly across the peritoneal membrane and require more time to equilibrate (20).
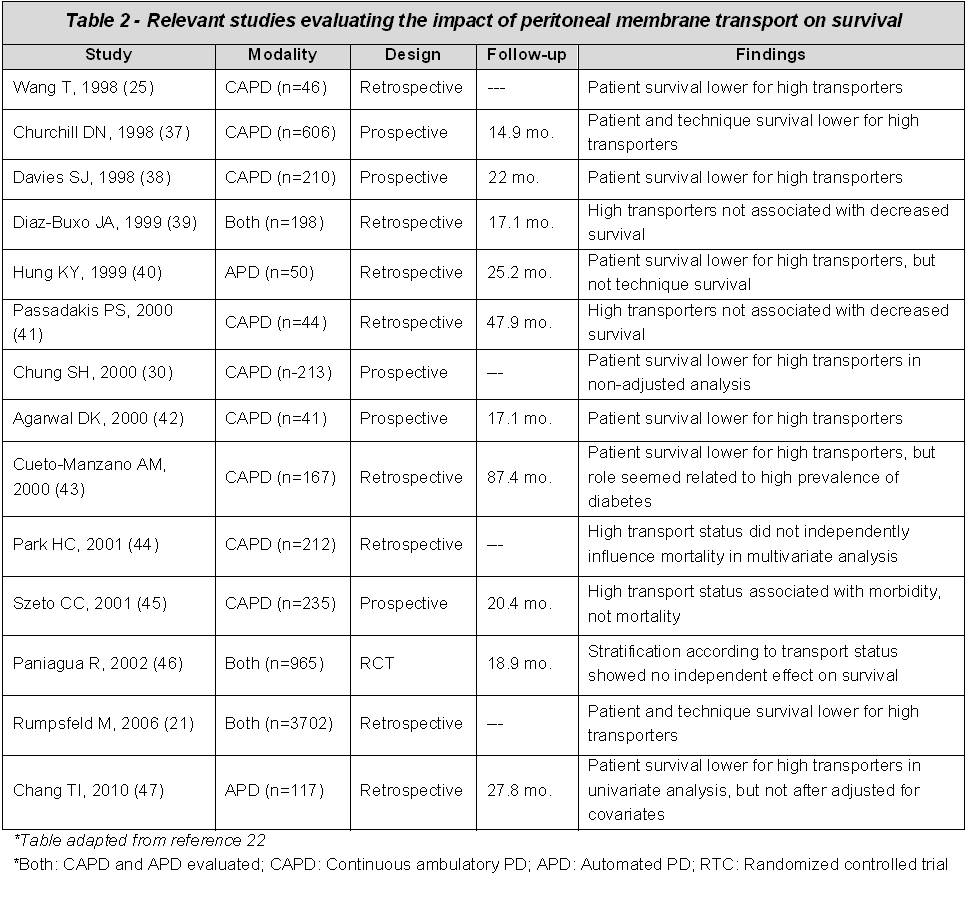
The purported benefit of prescribing APD in fast transporters is largely based on observational studies, many of which have reported poor survival with fast transporters using CAPD. Table 2, summarizes several studies that have evaluated the impact of transport status and PD modality.
 However, there is little direct evidence comparing the use of APD to CAPD in fast transporters. Of the studies that have been conducted, most tend to be underpowered or provide limited information on the PD regimen used. Despite the notion that fast transporters might be better suited for APD, drawing conclusions on the impact of modality on patient outcome has several inherent limitations. For one, patients using APD usually receive a greater number of exchanges per day and tend to use higher dialysate volumes. As such, these patients often receive a higher dialysate dose. Independent from dialysis modality and dose, data also suggests that fast transporters tend to have more peritoneal inflammation (23,24), poorer nutritional status (25, 26-29), and other comorbidities which may lead to worse outcomes (30).
However, there is little direct evidence comparing the use of APD to CAPD in fast transporters. Of the studies that have been conducted, most tend to be underpowered or provide limited information on the PD regimen used. Despite the notion that fast transporters might be better suited for APD, drawing conclusions on the impact of modality on patient outcome has several inherent limitations. For one, patients using APD usually receive a greater number of exchanges per day and tend to use higher dialysate volumes. As such, these patients often receive a higher dialysate dose. Independent from dialysis modality and dose, data also suggests that fast transporters tend to have more peritoneal inflammation (23,24), poorer nutritional status (25, 26-29), and other comorbidities which may lead to worse outcomes (30).
Using Transport Status to Further Optimize Dwell Time
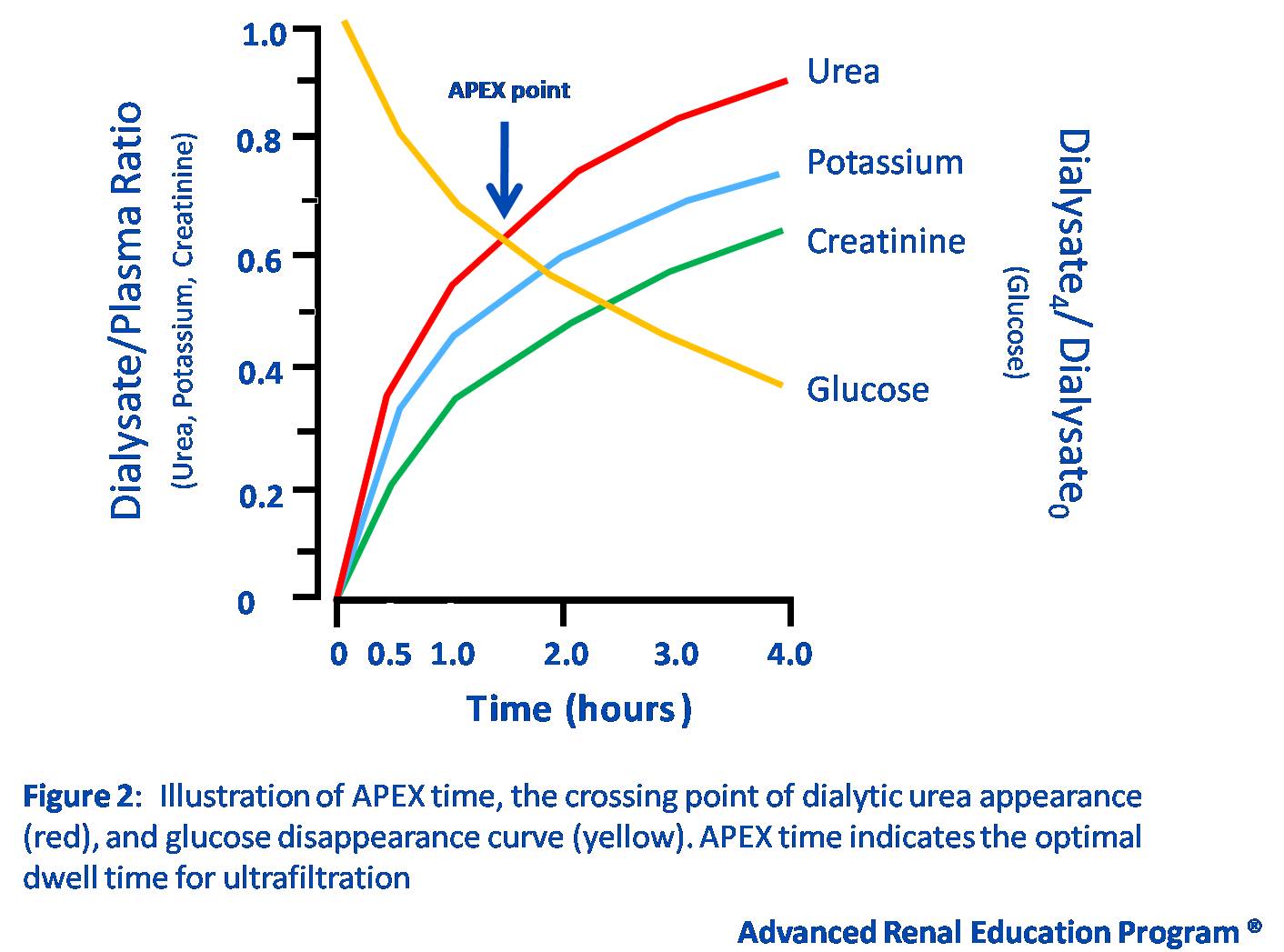
As stated above, fast transporters benefit from APD because the shorter dwell time helps maintain the osmotic gradient necessary for ultrafiltration. One method to obtain an approximate measure for the optimal dwell time for an individual patient is the determination of the accelerated peritoneal examination (APEX) point. As shown in Figure 2, this point describes the peritoneal permeability for both glucose and urea using equilibration curves obtained from a standard PET. The time at which the two curves cross is termed the APEX point, and indicates the optimal dwell time needed to maximize ultrafiltration (6-8,31). The shorter the APEX time, the higher the peritoneal permeability, and vice-versa. However, it should be noted that the APEX point has been best studied in children, and few studies have validated it in adults.
Figure 2 – Accelerated Peritoneal Examination (APEX) Point
In addition to influencing the PD regimen, knowledge of a patient’s transporter status may also help determine the most appropriate osmotic agent. Specifically, using high glucose concentrations or icodextrin for longer PD dwells has been shown to induce a greater ultrafiltration response in fast transporters (32-35).
Conclusion
The transport characteristics of the peritoneal membrane play an important role in solute clearance and ultrafiltration. Thus, the ability to determine and classify these characteristics has become an important means of predicting the most effective PD modality (36). In general, patients with a high (fast) peritoneal transport status will benefit from APD modalities that use shorter dwell times, whereas patients with a low (slow) transport status will benefit from longer dwell modalities such as CAPD. However, in practice, patient lifestyle and other non-medical issues may influence the peritoneal prescription more than transport status alone.
References
- Twardowski ZJ, Nolph KO, Khanna R, Prowant BF, Ryan LP, Moore HL, Nielsen MP. Peritoneal equilibration test. Perit Dial Bull. 1987;7:138-147.
- Daugirdas JT, Blake PG, Ing TS. Handbook of Dialysis: Chapter 13. 4th ed. Philadelphia: Lippincott Williams & Wilkins, 2007. 330-337p.
- La Milia V. Peritoneal transport testing. J Nephrol. 2010 Nov-Dec;23(6):633-47. https://www.ncbi.nlm.nih.gov/pubmed/20540028
- Cueto-Manzano AM. Rapid solute transport in the peritoneum: physiologic and clinical consequences. Perit Dial Int. 2009 Feb;29 Suppl 2:S90-5. https://www.ncbi.nlm.nih.gov/pubmed/19270240
- Agrawal A, Nolph KD. Management of high peritoneal transporters. Perit Dial Int. 2000;20 Suppl 2:S160-5. https://www.ncbi.nlm.nih.gov/pubmed/10911663
- Verger C, Larpent L, Veniez G, et al. L’APEX…description et utilisation. Bull Dial Perit 1:36-40, 1991
- Fischbach M, Mengus L, Birmele B, et a/. Peritoneal dialysate plasma equilibration curves, crossing time for urea and glucose: a function of age in children. In: Khanna R, Nolph KD, Prowant BF, Twardowski ZJ, Oreopoulos DO, eds. Advances in peritoneal dialysis. Toronto: Peritoneal Dialysis Bulletin Inc., 7:262-5, 1991
- Molony D, Craig J, eds. Evidenced-based Nephrology. Chichester, West Sussex UK: Blackwell Publishing; c2009. Chapter 45, Salt and Water Balance in Peritoneal Dialysis; p. 488-489.
- Gotch FA, Lipps BJ, Keen ML, Panlilio F. Computerized urea kinetic modeling to prescribe and monitor delivered Kt/V (pKt/V, dKt/V) in peritoneal dialysis. Fresenius Randomized Dialysis Prescriptions and Clinical Outcome Study (RDP/CO). Adv Perit Dial. 1996;12:43-5. https://www.ncbi.nlm.nih.gov/pubmed/8865870
- Pannekeet MM, Imholz AL, Struijk DG, Koomen GC, Langedijk MJ, Schouten N, de Waart R, Hiralall J, Krediet RT. The standard peritoneal permeability analysis: a tool for the assessment of peritoneal permeability characteristics in CAPD patients. Kidney Int. 1995 Sep;48(3):866-75. https://www.ncbi.nlm.nih.gov/pubmed/7474677
- Rippe B, Stelin G, Haraldsson B. Computer simulations of peritoneal fluid transport in CAPD. Kidney Int. 1991 Aug;40(2):315-25. https://www.ncbi.nlm.nih.gov/pubmed/1942781
- Haraldsson B. Assessing the peritoneal dialysis capacities of individual patients. Kidney Int. 1995 Apr;47(4):1187-98. https://www.ncbi.nlm.nih.gov/pubmed/7783418
- Rocco MV, Jordan JR, Burkart JM. Determination of peritoneal transport characteristics with 24-hour dialysate collections: dialysis adequacy and transport test. J Am Soc Nephrol. 1994 Dec;5(6):1333-8. https://www.ncbi.nlm.nih.gov/pubmed/7893998
- Twardowski ZJ. PET–a simpler approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial. 1990;6:186-91. https://www.ncbi.nlm.nih.gov/pubmed/1982805
- Diaz-Buxo JA. Peritoneal dialysis: matching the prescription to the membrane. Adv Perit Dial. 1999;15:96-100. https://www.ncbi.nlm.nih.gov/pubmed/10682080
- Li PK, Chow KM. Maximizing the success of peritoneal dialysis in high transporters. Perit Dial Int. 2007 Jun;27 Suppl 2:S148-52. https://www.ncbi.nlm.nih.gov/pubmed/17556294
- Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, Kawaguchi Y, Kawanishi H, Korbet S, Krediet R, Lindholm B, Oreopoulos D, Rippe B, Selgas R. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20 Suppl 4:S5-21. https://www.ncbi.nlm.nih.gov/pubmed/11098926
- Gokal R, Davies S, Coles G. Renal Association (UK) Standards Document. Vol. 3rd Edition, Suffolk: Royal College of Physicians of London, 2002.
- Dombros N, Dratwa M, Feriani M, Gokal R, Heimbürger O, Krediet R, Plum J, Rodrigues A, Selgas R, Struijk D, Verger C; EBPG Expert Group on Peritoneal Dialysis. European best practice guidelines for peritoneal dialysis. 6 Automated peritoneal dialysis. Nephrol Dial Transplant. 2005 Dec;20 Suppl 9:ix21-ix23. https://www.ncbi.nlm.nih.gov/pubmed/16263747
- Bammens B. Urea and uremic solutes: how does peritoneal dialysis work? Semin Nephrol. 2011 Mar;31(2):127-37.
- Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol. 2006 Jan;17(1):271-8. https://www.ncbi.nlm.nih.gov/pubmed/16306167
- Brimble KS, Walker M, Margetts PJ, Kundhal KK, Rabbat CG. Meta-analysis: peritoneal membrane transport, mortality, and technique failure in peritoneal dialysis. J Am Soc Nephrol. 2006 Sep;17(9):2591-8. Epub 2006 Aug 2. https://www.ncbi.nlm.nih.gov/pubmed/16885406
- Sezer S, Tutal E, Arat Z, Akçay A, Celik H, Ozdemir FN, Haberal M. Peritoneal transport status influence on atherosclerosis/inflammation in CAPD patients. J Ren Nutr. 2005 Oct;15(4):427-34. https://www.ncbi.nlm.nih.gov/pubmed/16198934
- Pecoits-Filho R, Araújo MR, Lindholm B, Stenvinkel P, Abensur H, Romão JE Jr, Marcondes M, De Oliveira AH, Noronha IL. Plasma and dialysate IL-6 and VEGF concentrations are associated with high peritoneal solute transport rate. Nephrol Dial Transplant. 2002 Aug;17(8):1480-6. https://www.ncbi.nlm.nih.gov/pubmed/12147798
- Wang T, Heimbürger O, Waniewski J, Bergström J, Lindholm B. Increased peritoneal permeability is associated with decreased fluid and small-solute removal and higher mortality in CAPD patients. Nephrol Dial Transplant. 1998 May;13(5):1242-9. https://www.ncbi.nlm.nih.gov/pubmed/9623561
- Nolph KD, Moore HL, Prowant B, Twardowski ZJ, Khanna R, Gamboa S, Keshaviah P. Continuous ambulatory peritoneal dialysis with a high flux membrane. ASAIO J. 1993 Oct-Dec;39(4):904-9. https://www.ncbi.nlm.nih.gov/pubmed/8123925
- Heaf J. CAPD adequacy and dialysis morbidity: detrimental effect of a high peritoneal equilibration rate. Ren Fail. 1995 Sep;17(5):575-87. https://www.ncbi.nlm.nih.gov/pubmed/8570870
- Kagan A, Bar-Khayim Y, Schafer Z, Fainaru M. Heterogeneity in peritoneal transport during continuous ambulatory peritoneal dialysis and its impact on ultrafiltration, loss of macromolecules and plasma level of proteins, lipids and lipoproteins. Nephron. 1993;63(1):32-42. https://www.ncbi.nlm.nih.gov/pubmed/8446249
- Cueto-Manzano AM, Gamba G, Correa-Rotter R. Peritoneal protein loss in patients with high peritoneal permeability: comparison between continuous ambulatory peritoneal dialysis and daytime intermittent peritoneal dialysis. Arch Med Res. 2001 May-Jun;32(3):197-201. https://www.ncbi.nlm.nih.gov/pubmed/11395184
- Chung SH, Chu WS, Lee HA, Kim YH, Lee IS, Lindholm B, Lee HB. Peritoneal transport characteristics, comorbid diseases and survival in CAPD patients. Perit Dial Int. 2000 Sep-Oct;20(5):541-7. https://www.ncbi.nlm.nih.gov/pubmed/11117245
- Fischbach M, Lahlou A, Eyer D, Desprez P, Geisert J. Determination of individual ultrafiltration time (APEX) and purification phosphate time by peritoneal equilibration test: application to individual peritoneal dialysis modality prescription in children. Perit Dial Int. 1996;16 Suppl 1:S557-60. https://www.ncbi.nlm.nih.gov/pubmed/8728270
- Wolfson M, Piraino B, Hamburger RJ, Morton AR; Icodextrin Study Group. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002 Nov;40(5):1055-65. https://www.ncbi.nlm.nih.gov/pubmed/12407652
- Araújo Teixeira MR, Pecoits-Filho RF, Romão Junior JE, Sabbaga E, Marcondes MM, Abensur H. The relationship between ultrafiltrate volume with icodextrin and peritoneal transport pattern according to the peritoneal equilibration test. Perit Dial Int. 2002 Mar-Apr;22(2):229-33. https://www.ncbi.nlm.nih.gov/pubmed/11990408
- Wiggins KJ, Rumpsfeld M, Blizzard S, Johnson DW. Predictors of a favourable response to icodextrin in peritoneal dialysis patients with ultrafiltration failure. Nephrology (Carlton). 2005 Feb;10(1):33-6. https://www.ncbi.nlm.nih.gov/pubmed/15705179
- Qi H, Xu C, Yan H, Ma J. Comparison of icodextrin and glucose solutions for long dwell exchange in peritoneal dialysis: a meta-analysis of randomized controlled trials. Perit Dial Int. 2011 Mar-Apr;31(2):179-88. https://www.ncbi.nlm.nih.gov/pubmed/21119069
- Chung SH, Heimbürger O, Lindholm B. Poor outcomes for fast transporters on PD: the rise and fall of a clinical concern. Semin Dial. 2008 Jan-Feb;21(1):7-10. https://www.ncbi.nlm.nih.gov/pubmed/18251948
- Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Pagé D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998 Jul;9(7):1285-92. https://www.ncbi.nlm.nih.gov/pubmed/9644640
- Davies SJ, Phillips L, Russell GI. Peritoneal solute transport predicts survival on CAPD independently of residual renal function. Nephrol Dial Transplant. 1998 Apr;13(4):962-8. https://www.ncbi.nlm.nih.gov/pubmed/9568858
- Diaz-Buxo JA, Lowrie EG, Lew NL, Zhang SM, Zhu X, Lazarus JM. Associates of mortality among peritoneal dialysis patients with special reference to peritoneal transport rates and solute clearance. Am J Kidney Dis. 1999 Mar;33(3):523-34. https://www.ncbi.nlm.nih.gov/pubmed/10070917
- Hung KY, Lin TJ, Tsai TJ, Chen WY. Impact of peritoneal membrane transport on technique failure and patient survival in a population on automated peritoneal dialysis. ASAIO J. 1999 Nov-Dec;45(6):568-73. https://www.ncbi.nlm.nih.gov/pubmed/10593688
- Passadakis PS, Thodis ED, Panagoutsos SA, Selisiou CA, Pitta EM, Vargemezis VA. Outcome for continuous ambulatory peritoneal dialysis patients is not predicted by peritoneal permeability characteristics. Adv Perit Dial. 2000;16:2-6. https://www.ncbi.nlm.nih.gov/pubmed/11045251
- Agarwal DK, Sharma AP, Gupta A, Sharma RK, Pandey CM, Kumar R, Masih SP. Peritoneal equilibration test in Indian patients on continuous ambulatory peritoneal dialysis: does it affect patient outcome? Adv Perit Dial. 2000;16:148-51. https://www.ncbi.nlm.nih.gov/pubmed/11045281
- Cueto-Manzano AM, Correa-Rotter R. Is high peritoneal transport rate an independent risk factor for CAPD mortality? Kidney Int. 2000 Jan;57(1):314-20. https://www.ncbi.nlm.nih.gov/pubmed/10620214
- Park HC, Kang SW, Choi KH, Ha SK, Han DS, Lee HY. Clinical outcome in continuous ambulatory peritoneal dialysis patients is not influenced by high peritoneal transport status. Perit Dial Int. 2001;21 Suppl 3:S80-5. https://www.ncbi.nlm.nih.gov/pubmed/11887869
- Szeto CC, Law MC, Wong TY, Leung CB, Li PK. Peritoneal transport status correlates with morbidity but not longitudinal change of nutritional status of continuous ambulatory peritoneal dialysis patients: a 2-year prospective study. Am J Kidney Dis. 2001 Feb;37(2):329-36. https://www.ncbi.nlm.nih.gov/pubmed/11157374
- Paniagua R, Amato D, Vonesh E, Correa-Rotter R, Ramos A, Moran J, Mujais S; Mexican Nephrology Collaborative Study Group. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002 May;13(5):1307-20. https://www.ncbi.nlm.nih.gov/pubmed/11961019
- Chang TI, Park JT, Lee DH, Lee JH, Yoo TH, Kim BS, Kang SW, Lee HY, Choi KH. High peritoneal transport status is not an independent risk factor for high mortality in patients treated with automated peritoneal dialysis. J Korean Med Sci. 2010 Sep;25(9):1313-7. https://www.ncbi.nlm.nih.gov/pubmed/20808674
P/N 101705-01 Rev A 02/2012