Albumin Status in Dialysis Patients
Abstract
Serum albumin (sAlb) is a widely used biomarker of nutritional status and persistent inflammation in dialysis patients. Albumin levels are lower in dialysis patients than in the general population. Additionally, hypoalbuminemia (sAlb<3.5g/dl) is strongly associated with mortality, increased hospitalization rates, and cost related with hospitalization in hemodialysis patients. The National Kidney Foundation KDOQI guidelines recommend that serum albumin (sAlb) levels in hemodialysis patients be maintained above 4.0 g/dL. The concentration of serum albumin is the net result of its synthesis, breakdown, body pool size, volume of distribution, and exchange between intra- and extra-vascular spaces, as well as losses from the body. The predominant cause of hypoalbuminemia in dialysis patients is a decrease in the albumin synthesis rate (due to protein-calorie malnutrition) and an increase in fractional catabolic rate (FCR), both driven by acute phase response. Additionally, hypoalbuminemia can also result from redistribution of albumin pools or from albumin losses. During HD treatments, the extracorporeal albumin loss with low flux (LF) and high flux (HF) membranes is negligible. However, recently the use of membranes with large pore size and increased permeability such as the Medium cut off (MCO) and High cut off (HCO) dialyzers, have been associated with elevated albumin loss along with the good clearance of large middle molecules. Any additional albumin loss is of concern in the dialysis population, where albumin levels are already below targets. Although the acceptable upper limit of extracorporeal albumin loss per treatment remains unknown, additional long-term controlled studies are needed to evaluate whether the beneficial effects of enhanced (larger) middle molecule removal with high permeable membranes outweigh the potential adverse effects of increased albumin loss on patient outcomes.
Introduction
Hypoalbuminemia in dialysis is a highly prevalent condition associated with increased morbidity and mortality. Importantly, many factors contribute to hypoalbuminemia in ESKD on HD, including protein energy wasting, inflammation, volume expansion, renal loss and loss into the dialysate (1,2). Additionally, the loss of albumin in the dialysate may further aggravate the existing hypoalbuminemia in the patient on HD. Serum albumin concentration is a function of its rate of synthesis by the liver (12 g/day), the fractional catabolic rate (FCR, the fraction of the vascular pool catabolized/unit of time) (~4% daily), external loss (renal, gastro-intestinal and transmembrane loss during dialysis), hydration status and redistribution from the vascular to the extravascular space (or vice versa). The factors regulating serum albumin are similar between individuals with and without CKD. Individuals with hypoalbuminemia and advanced CKD have plasma albumin half-lives and degradation rates similar to those of healthy individuals (3),with possibly even higher rates of albumin synthesis (4) and turnover (5).
Range of serum albumin levels in U.S. dialysis patients
The National Kidney Foundation KDOQI guidelines recommend that serum albumin (sAlb) levels in hemodialysis patients be maintained above 4.0 g/dL (6). However, many studies report HD patients have serum albumin levels below the recommended range (> 4g/dL).
- Lowrie and Lew reported a mean sAlb of 3.8 g/dL in 12,000 HD patients assessed between 1987-88 (7)
- A large study of 36,757 incident hemodialysis patients reported baseline mean sAlb levels of 3.57 ±0.46 g/dL (8)
- Ye et al. reported that in 110,794 HD patients, the mean sAlb was 3.5 ± 0.5 g/dL (9)
- In May 2017, 18.9% of HD and 44.3% of PD patients were hypoalbuminemic with levels <3.5 g/dL (10)
- Kalantar-Zadeh et al. reported that sAlb levels were <4.0 g/dL in 72% of maintenance hemodialysis (MHD) patients (N= 58,058); 52% had sAlb levels <3.8 g/dL (11)
- The 2018 USRDS reported some improvement where 1% of HD patients had sAlb levels <4.0 g/dL, and 18.9% below 3.5 g/dL (12)
Effect of low serum albumin on patient outcomes in HD patients
Serum albumin (sAlb) is considered as a marker of mortality and inflammation (13,14). Continual loss of large quantities of albumin could lead to hypoalbuminemia and malnutrition, especially in patients whose liver cannot counterbalance the potential losses by various mechanisms including the loss across the dialyzer. Serum albumin lower than 4 g/dL has long been associated with an increased risk of morbidity, mortality, and increase in hospitalization rates and subsequent increase in the cost for patients on dialysis.
Hospitalization rates and cos
Regarding hospitalization rates and cost, compared to the patients with serum albumin levels ≥ 4 g/dL who have an average hospital stay of 11.94 days per year, dialysis patients with serum albumin levels of < 3.0 g/dL spent 7.8 days, 3.0 to 3.49 g/dL spent 7.6 days, and 3.5 to 3.99 g/dL spent 3.98 days in excess hospital days each year (15) . According to USRDS, the cost of hospitalization for a dialysis patient is approximately $15,907.18 for the average length of stay (11.3 days). Based on the stated information, an estimated calculation of hospital spending based on the excess hospital days (data from Rocco et al. (15)); reduced albumin levels may result in increased hospital spending on average of $5,602-$10,980 per patient per year.
Ikizler and colleagues followed 73 adult patients for 15 months from a single outpatient center in the US who had been on hemodialysis for at least three months (16). When assessed as a single variable, decreased serum albumin correlated with increased hospitalization rates (>4.1 mg/dL: 17%, 4.1-3.9 mg/dL: 24%, 3.9-3.7 mg/dL: 27%, and <3.7 mg/dL: 43%, p=0.05 by regression analysis). However, in a multivariate analysis that included serum c-reactive protein, reactance (via bioelectrical impedance), and albumin there was no significant associated between albumin levels and hospitalization risk.
Mortality
The relationship between albumin and mortality in long-term dialysis patients was first described by Goldwasser and colleagues in 1993 with a small (184 patients), prospective study that sought to find relationships between serum biochemical markers and mortality risk (17). In patients (n=108) who had been on dialysis for ≥ 12 months, low serum albumin (<3.5 g/dL) was the strongest predictor of mortality. Specifically, serum albumin levels below 3.5 g/dL were associated with a seven-fold increase in mortality risk compared to levels above 3.5 g/dL.
In a randomized study, Locatelli F et al. (18), (N=647) compared the impact of membrane permeability (high- vs. low-flux) on survival in incident HD patients treated with a minimum spKt/V of 1.2. They also stratified patients by low (≤4 g/dL) or normal serum albumin levels (>4 g/dL). The total number of deaths observed in this study was 162, of which 132 events occurred in patients with serum albumin ≤4 g/dL: unadjusted mortality of 27% in those with low sAlb vs. 19.5% in those with >4 g/dL). The adjusted relative risk reduction of mortality due to high- vs. low-flux dialyzers in patients with low sAlb was 37%. There was no significant difference in the mortality of patients with sAlb >4 g/dL (n= 154; 30 deaths) between high-flux and the low-flux groups via Kaplan-Meir analysis (P=0.211).
In addition, several observational studies over the past 3-4 decades have reported associations of low sAlb with poor outcomes in hemodialysis patients (19–23). However, as with all observational and/or retrospective studies, there are numerous confounders that prevent cause and effect relationships from being determined. Brief summaries of some of these studies are listed below.
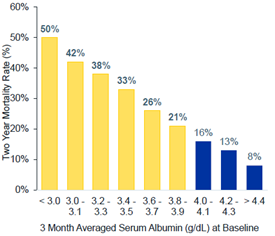
- In 12,000 HD patients, Lowrie and Lew (7) determined that the adjusted relative risk of death increased geometrically and markedly as the sAlb concentration decreased below the range of 4.0-4.5 g/dl. The relative risk increased to approximately 2.2 with a sAlb of 3.5-4.0 g/dL; to 6.7 with a sAlb of 3.0-3.5 g/dL; to 15.3 with a sAlb of 2.5-3.0 g/dL; and to 18.5 with a sAlb below 2.5 g/dL. After age of the patients, sAlb was the most powerful predictor of mortality in this analysis.
- Owen and colleagues conducted a retrospective cohort study of over 13,000 hemodialysis patients from October 1, 1990 through March 31, 1991 from a single large dialysis organization in the United States (24). As shown in Table 1 below, the mortality odds ratio increased as serum album decreased.
Table 1 Adjusted Risk of Death According to Serum Albumin Concentration (24)
| Serum Albumin (g/dL) | Odds Ratio | 95% Confidence Interval | P Value |
| ≥ 4.5 | 0.47 | 0.33 – 0.66 | 0.03 |
| 4.0 – 4.4 (reference group) | 1.00 | – | – |
| 3.5 – 3.9 | 1.48 | 1.37 – 1.59 | <0.001 |
| 3.0 – 3.4 | 3.13 | 2.87 – 3.41 | <0.001 |
| 2.5 – 2.9 | 7.08 | 6.12 – 8.19 | <0.001 |
| <2.5 | 12.8 | 9.62 – 16.97 | <0.001 |
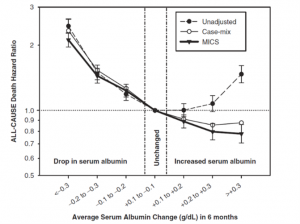
However, Kalantar-Zadeh et al. (11) investigated the time-dependent multivariate models and examined whether changes in the serum albumin over time may be a better marker for predicting survival in HD patients independent of baseline serum albumin and other covariates. The authors concluded that time-varying hypoalbuminemia predicts all-cause and CV death differently from fixed measures of serum albumin in HD patients. An increase in serum albumin over time is associated with better survival independent of baseline serum albumin or other malnutrition–inflammation complex syndrome (MICS) surrogates. Based on the association, the authors predicted that an intervention that could increase serum albumin to levels above 3.8 g/dl might reduce the number of HD deaths in the USA by ∼10 000 annually. A subset of sample from DOPPS study consisting of 7719 hemodialysis patients from 145 dialysis facilities in the United States were evaluated for the mortality risk and changes in nutritional indicators (25). A strong inverse association was observed between mortality and the serum albumin concentration. Mortality risk also was strongly associated with a decline in the serum albumin concentration during the six-month follow-up. A decline in the serum albumin concentration of greater than 5.3% was associated with a near doubling of mortality risk (P < 0.001).
Figure 1: Two-year, all-cause mortality rate and Impact of Serum Albumin Changes on Subsequent Mortality Risk
 |
 |
Mukai et al. suggests that although low sAlb is commonly found in dialysis patients and associated with poor outcomes, it may be more a marker of persistent inflammation than one of nutritional assessment (26). In support, Mutsert et al. reported that the association between sAlb and mortality can be partly explained by inflammatory effects and not nutrition (27).Thijssen and colleagues also reported that sAlb is dependent upon interactions with inflammation, nutrition, and dialysis efficacy (28).
Albumin is a clinically actionable maker that can be improved by medical/nutritional intervention.
The correlations described above suggest that increasing sAlb might improve mortality; however, few studies of good quality have been performed to investigate this. A summary of studies identified is provided below.
Randomized Clinical Trials and Systematic Reviews
In a pilot study, Hristea et al. investigated the feasibility and the effects of a 6-month intradialytic cycling program combined with nutritional support on PEW, physical functioning (gait, balance, muscle strength) and quality of life (QoL) in older HD patients (mean age 69.7 ± 14.2 years) (29). Twenty-one patients fulfilling diagnostic criteria of PEW were randomly assigned to Nutrition-Exercise group (GN-Ex, n = 10) or Nutrition group (GN, n = 11). Both groups received nutritional supplements in order to reach recommended protein and energy intake goals. No significant difference between groups was found in the number of patients having reached remission of PEW. Likewise, no changes in serum-albumin, -prealbumin, C-reactive protein, body mass index, lean- and fat-tissue index, or quadriceps force were observed.
A systematic review by Sigrist et al. assessed studies on parenteral nutrition in hemodialysis patients conducted up to July 2009 (30). They determined that the clinical data is insufficient to demonstrate a net benefit or net harm of IDPN in hemodialysis patients. Of those trials assessed that reported albumin levels, Cano et al. reported that albumin levels increased 84 days after starting the regimen (PDPN, 0.95 ± 2.19; Control subjects, -0.43 ± 2.36 P < .05; mean change from baseline 1.38 g/dL) (31); Dezfuli et al. reported that 72% of patients (N=196) responded to IDPN with an average increase in albumin of 0.4 g/dL (32); Navarro et al. (33) reported that after 3 months of administration of amino acids, the median serum albumin concentration increased significantly compared with baseline: 42.2 g/L (range: 38–44 g/L) compared with 36.2 g/L (34.5–40.7 g/L) (P < 0.05); no changes were seen in the control group.
Observational Studies
In a large prospective study (N=860), Sherman et al. reported that the IDWG in maintenance HD patients correlated directly with parameters of improved nutrition, including edema-free body weight, normalized PNA, and serum albumin concentrations. Compared with patients having <2 kg IDWG (n = 378), patients with >3 kg IDWG (n = 138) weighed more (dry weight, 76.8 v 61.7 kg), had higher nPCR (1.15 vs. 0.96 g/kg/d), and had higher serum albumin levels (3.96 vs 3.79 g/dL) (all P < 0.001). When IDWG was assessed as a function of dry weight, patients with IDWG >4.5% of dry weight (n = 151) had higher nPCR (1.17 v 0.94 g/kg/d) but weighed less (60.1 vs. 70.0 kg) than patients with IDWG < 3% of dry weight (n = 355) (all P < 0.001). The authors suggest better nutrition and increased appetite in patients with higher IDWG.
Capelli et al. retrospectively compared 50 malnourished maintenance hemodialysis patients receiving intradialytic parenteral nutrition (IDPN) to 31 similar patients who had not received IDPN. The mortality rate was lower in the IDPN treated patients.
In another retrospective study by Chertow et al., 1679 MHD patients treated for one year with IDPN were evaluated; 517 patients who did not receive IDPN served as the control group (34). The IDPN-treated and control patients were grouped according to their serum albumin levels, and data were adjusted for predialysis serum creatinine concentrations and other characteristics. The IDPN patients were slightly older (62 vs. 58 years), more often white (53 vs. 47%), diabetic (41 vs. 35%), had lower serum albumin (3.25 vs. 3.78 g/dl) and creatinine (9.3 vs. 12.0 mg/dl), and had a slightly higher dialysis dose (URR, 61.1 vs 60.0%). The results indicated that for those patients with serum albumin concentrations of ≤3.3 g/dL, the odds ratio of death was significantly reduced in the individuals treated with IDPN vs. those who were not. The odds ratio of death in the lDPN vs. the control patients was ~0.73 when the serum albumin was 3.3 g/dL or lower, ~62 when the serum albumin was either 3.2 or 3.1 g/dL or lower, and ~0.58 when the serum albumin was 3.0 g/dL or lower. Despite the study limitations, the authors concluded that the improvement in survival at year’s end among patients with serum albumin ≤ 3.4 g/dL suggests that malnutrition and its attendant ill effects in hemodialysis patients may respond to aggressive therapeutic intervention, such as IDPN.
Bossola et al. assessed appetite and nutritional parameters in 59 HD patients (35). SAlb levels were significantly lower in patients with a fair and poor/ very poor appetite than in patients with a very good or good appetite (P ≤0.0001).
Albumin loss with different dialyzers
Various studies have shown that the average loss of albumin during hemodialysis using conventional high flux dialyzers is minimal, ranging from being undetectable up to ~1.2 g/session in the spent dialysate for most high flux dialyzers (36,37,46,38–45). Recently, with the use of high-cutoff membranes (HDO) and medium-cutoff membranes (MCO) (also known as protein-leaking membranes (47)), increased clearances of low molecular weight proteins and middle molecules have been reported (48). However, these membranes allow substantial albumin loss into the dialysate during a 4-hr HD treatment compared with conventional HD using high-flux dialyzers. Several HCO or high-performance (HPM) dialyzer membranes used in studies in Japan and MCO, such as Theranova 400 or 500, do allow albumin leakage up to 10 g/session (49) and 3.2 g/session, respectively (50–52). The impact of such continual loss of albumin over time on patient outcomes is not yet clear, and the upper limit of albumin loss across dialyzers has not been determined (14). For instance, large amounts of albumin loss have been associated with hypotension, post dialysis fatigue, decrease in serum albumin levels due to long-term use, elevation of total cholesterol values, and detrimental effects on lipid metabolism, large individual differences have been pointed out in regard to tolerance of albumin loss (14).
Albumin loss >3.4 g/4-h treatment with high permeable membranes has been associated with a decrease in serum albumin levels within 2–3 weeks after treatment initiation, suggesting that albumin loss may be too high to be compensated for by an increase in albumin synthesis and/or altered distribution. However, studies did not control for malnutrition and inflammation, although inflammation-augmented albumin catabolism may be relatively low with these membranes due to enhanced removal of proinflammatory cytokines.
Conversely, high-flux dialyzers are generally associated with initial increases in serum albumin concentrations (40,53–59), possibly due to fluid removal and volume concentration. In a study of 976 patients receiving in-center HD with one of 4 high flux dialyzers (Optiflux F160NR, F180NR, F200NR, or F250NR), pre-HD albumin levels demonstrated small but statistically significant increases over 6 months (0.05 g/dL to 0.11 g/dL). When the analysis was limited to those 156 patients with hypoalbuminemia (≤3.5 g/dL) at baseline, a mean increase in serum albumin of 0.31 g/dL (0.34, 0.29 and 0.28 g/dL with the F160NR, F180NR, and F200NR/F250NR dialyzers, respectively) was observed (58). The increases in serum albumin levels observed with high flux dialyzer use persist. In a study of 30 HD patients switched from low flux dialyzers to high-flux (FX60) dialyzers, Li and colleagues observed significant increases in serum albumin levels after 3 years of use following non-significant increases at 1 year (59).
Thus, dialyzers that increase large solute removal at the expense of albumin loss could adversely impact patients, increasing potential risk of hypoalbuminemia; nonetheless, the amount of albumin losses that exaggerate hypoalbuminemia leading to unfavorable patient outcomes remains unknown.
There are multiple caveats to all studies cited above, including, but not limited to, that most of the studies included small sample sizes over short durations. Use of patient-reported parameters and no control or comparative groups in some studies can skew the data and conclusions. Many are also observational in nature with inherent biases (e.g., selection bias, inconsistent protocols, missing data, etc.) and used different methods for albumin measurement and reporting. Thus, making definitive conclusions on outcomes, based on available published data, is difficult.
Although, the acceptable upper limit of extracorporeal albumin loss per treatment remains unknown, the abovementioned data suggest being cautious with the use of dialyzers with highly permeable membranes that produce losses of albumin. According to USRDS, 68.1% of HD patients have sAlb levels <4.0 g/dL, and 18.9% below 3.5 g/dL (13) with an increased mortality rate (25). Thus, an additional albumin loss with the use of high permeable dialyzers may potentially aggravate the mortality risk associated with already low albumin levels. Nonetheless, some studies suggest that some albumin loss is tolerable, and HCO and MCO membranes can help to improve anemia, reduce plasma levels of pentosidine and homocysteine concentrations, as well as reduce potentially toxic glycosylated and oxidized proteins (35-60 kD range) in the plasma of dialysis patients (60). Galli et al. reported stable serum albumin levels despite albumin losses of 2–5 g per treatment, but these results need to be confirmed in studies of longer duration (61). The quantity of albumin loss that is tolerable has not been definitively determined (60), but Kawanishi et al. suggested that a ‘desirable albumin leakage in one treatment should be less than 4 g (62).
The argument for the above rationale stems from the following:
- In individuals with normal renal function, ~10 g of albumin is filtered in the glomerulus per day. Some of this is reabsorbed as amino acids and re-synthesized into albumin in the liver. However, >1.3 g of the filtered albumin fragments are excreted per day (63).
- Sera from hemodialysis patients have shown modified forms of albumin (64,65).
- Kaysen et al. showed that a decrease in albumin synthesis, rather than increased albumin loss, is the principal cause of the low serum albumin in hypoalbuminemic hemodialysis patients (66), and this defect may be related to the presence of malnourished and/or inflammatory states (1,61).
- Many patients can compensate for moderate albumin losses by increasing its synthesis in the liver (67).
- Albumin—either as a carrier protein for small, highly protein-bound uremic toxins (8,46) or in a modified form (5,6,47)—could play a role in causing uremic toxicity (64,65,68–70).
As such, Tsuchida (71) suggests that in dialysis patients, biologically active, potentially toxic molecules that are bound to albumin or the oxidized form of albumin devoid of activity cannot be filtered by the kidney and would therefore accumulate. Therefore, potential benefits derived from albumin leakage across a dialyzer would involve removal of these substances, which could facilitate synthesis of new albumin with an antioxidant effect.
Conclusion
Most HD patients have serum albumin levels below the recommended range (> 4g/dL) by the National Kidney Foundation KDOQI guidelines. The data presented here suggest HD patients with serum albumin < 4 g/dL may be associated with an increased risk of mortality, have a higher hospitalization rate and cost associated with that. The average loss of albumin during hemodialysis using conventional high-flux dialyzers is minimal. The use of MCO or HCO dialyzer membranes (also known as protein-leaking membranes) are associated with the increased clearance of middle and large middle molecule, such as proteins. However, these membranes allow substantial albumin loss into the dialysate during a 4-hr HD treatment compared with conventional HD using high-flux dialyzers. Although greater middle molecule removal has been shown to be a surrogate for clinical benefits, such as improvement in restless legs symptoms, nutritional score, and quality of life outcomes, studies using HCO and MCO membrane dialyzers have not demonstrated these clinical benefits. Further randomized trials with clinical outcomes will be required to determine whether the clearance of the middle molecules along with higher albumin loss than the conventional dialyzers would be safer and result in any clinically meaningful differences for patients treated with protein-leaking dialyzers.
References:
- Kaysen GA, Dubin JA, Müller H-G, Mitch WE, Rosales LM, Levin NW. Relationships among inflammation nutrition and physiologic mechanisms establishing albumin levels in hemodialysis patients. Kidney Int. 2002;61(6):2240-2249. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12028466.
- Yeun JY, Kaysen GA. Factors influencing serum albumin in dialysis patients. Am J Kidney Dis. 1998;32(6 Suppl 4):S118-25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9892378.
- Fleck A, Hawker F, Wallace PI, Raines G, Trotter J, Ledingham IM, Calman KC. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;325(8432):781-784.
- Prinsen BH, Rabelink TJ, Beutler JJ, Kaysen GA, De Boer J, Boer WH, Hagen EC, Berger R, De Sain-Van Der MGM. Increased albumin and fibrinogen synthesis rate in patients with chronic renal failure. Kidney Int. 2003;64(4):1495-1504.
- Kaysen GA, Schoenfeld PY. Albumin homeostasis in patients undergoing continuous ambulatory peritoneal dialysis. Kidney Int. 1984;25(1):107-114. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6727122.
- KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. NKF KDOQI Guidel. 2000. Available from: http://www2.kidney.org/professionals/KDOQI/guidelines_nutrition/doqi_nut.html.
- Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15(5):458-482. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2333868.
- Eriguchi R, Obi Y, Streja E, Tortorici AR, Rhee CM, Soohoo M, Kim T, Kovesdy CP, Kalantar-Zadeh K. Longitudinal associations among renal urea clearance–corrected normalized protein catabolic rate, serum albumin, and mortality in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12(7):1109-1117.
- Ye X, Dekker MJE, Maddux FW, Kotanko P, Konings CJAM, Raimann JG, van der Sande FM, Usvyat LA, Kooman JP, Thijssen S. Dynamics of Nutritional Competence in the Last Year Before Death in a Large Cohort of US Hemodialysis Patients. J Ren Nutr. 2017;27(6):412-420.
- v2 CH2 Clinical Indicators and Preventive Care. Available from: https://www.usrds.org/2018/view/v2_02.aspx?zoom_highlight=albumin.
- Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20(9):1880-1888. Available from: https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfh941.
- v2 CH5 Mortality. Available from: https://www.usrds.org/2018/view/v2_05.aspx.
- Locatelli F, Karaboyas A, Pisoni RL, Robinson BM, Fort J, Vanholder R, Rayner HC, Kleophas W, Jacobson SH, Combe C, et al. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant. 2018;33(4):683-689. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29040687.
- Krieter DH, Canaud B. High permeability of dialysis membranes: what is the limit of albumin loss? Nephrol Dial Transplant. 2003;18(4):651-654. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12637630.
- Rocco M V, Soucie JM, Reboussin DM, McClellan WM. Risk factors for hospital utilization in chronic dialysis patients. Southeastern Kidney Council (Network 6). J Am Soc Nephrol. 1996;7(6):889-896. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8793798.
- Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: A prospective study. Kidney Int. 1999;55(5):1945-1951. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10231458.
- Goldwasser P, Mittman N, Antignani A, Burrell D, Michel MA, Collier J, Avram MM. Predictors of mortality in hemodialysis patients. J Am Soc Nephrol. 1993;3(9):1613-1622. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8507818.
- Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R, et al. Effect of Membrane Permeability on Survival of Hemodialysis Patients. J Am Soc Nephrol . 2009;20(3):645-654. Available from: http://jasn.asnjournals.org/content/20/3/645.abstract.
- Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J Am Soc Nephrol. 1996;7(5):728-736. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8738808.
- Agarwal R, Davis JL, Smith L. Serum Albumin Is Strongly Associated with Erythropoietin Sensitivity in Hemodialysis Patients. Clin J Am Soc Nephrol. 2008;3(1):98-104. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18045859.
- FUKUOKA K, NAKAO K, MORIMOTO H, NAKAO A, TAKATORI Y, ARIMOTO K, TAKI M, WADA J, MAKINO H. Glycated albumin levels predict long-term survival in diabetic patients undergoing haemodialysis. Nephrology. 2008;13(4):278-283. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18476915.
- Mehrotra R, Duong U, Jiwakanon S, Kovesdy CP, Moran J, Kopple JD, Kalantar-Zadeh K. Serum Albumin as a Predictor of Mortality in Peritoneal Dialysis: Comparisons With Hemodialysis. Am J Kidney Dis. 2011;58(3):418-428. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21601335.
- Moreau-Gaudry X, Jean G, Genet L, Lataillade D, Legrand E, Kuentz F, Fouque D. A simple protein-energy wasting score predicts survival in maintenance hemodialysis patients. J Ren Nutr. 2014;24(6):395-400. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1051227614001162.
- Owen WF, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The Urea Reduction Ratio and Serum Albumin Concentration as Predictors of Mortality in Patients Undergoing Hemodialysis. N Engl J Med. 1993;329(14):1001-1006. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8366899.
- Daugirdas JT, Depner TA, Inrig J, Mehrotra R, Rocco M V., Suri RS, Weiner DE, Greer N, Ishani A, MacDonald R, et al. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 Update. Am J Kidney Dis. 2015;66(5):884-930. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0272638615010197.
- Mukai H, Villafuerte H, Qureshi AR, Lindholm B, Stenvinkel P. Serum albumin, inflammation, and nutrition in end-stage renal disease: C-reactive protein is needed for optimal assessment. Semin Dial. June 2018. Available from: http://doi.wiley.com/10.1111/sdi.12731.
- de Mutsert R, Grootendorst DC, Indemans F, Boeschoten EW, Krediet RT, Dekker FW, Netherlands Cooperative Study on the Adequacy of Dialysis-II Study Group. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr. 2009;19(2):127-135. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1051227608003890.
- Thijssen S, Wystrychowski G, Usvyat L, Kotanko P, Levin NW. Determinants of serum albumin concentration analyzed in a large cohort of patients on maintenance hemodialysis. J Ren Nutr. 2007;17(1):70-74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17198937.
- Hristea D, Deschamps T, Paris A, Lefrançois G, Collet V, Savoiu C, Ozenne S, Coupel S, Testa A, Magnard J. Combining intra-dialytic exercise and nutritional supplementation in malnourished older haemodialysis patients: Towards better quality of life and autonomy. Nephrology. 2016;21(9):785-790. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26890997.
- Sigrist MK, Levin A, Tejani AM. Systematic Review of Evidence for the Use of Intradialytic Parenteral Nutrition in Malnourished Hemodialysis Patients. J Ren Nutr. 2010;20(1):1-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19788956.
- Cano N, Labastie-Coeyrehourq J, Lacombe P, Stroumza P, di Costanzo-Dufetel J, Durbec JP, Coudray-Lucas C, Cynober L. Perdialytic parenteral nutrition with lipids and amino acids in malnourished hemodialysis patients. Am J Clin Nutr. 1990;52(4):726-730. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2119558.
- Dezfuli A, Scholl D, Lindenfeld SM, Kovesdy CP, Kalantar-Zadeh K. Severity of hypoalbuminemia predicts response to intradialytic parenteral nutrition in hemodialysis patients. J Ren Nutr. 2009;19(4):291-297. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1051227609000454.
- Navarro JF, Mora C, León C, Martín-Del Río R, Macía ML, Gallego E, Chahin J, Méndez ML, Rivero A, García J. Amino acid losses during hemodialysis with polyacrylonitrile membranes: effect of intradialytic amino acid supplementation on plasma amino acid concentrations and nutritional variables in nondiabetic patients. Am J Clin Nutr. 2000;71(3):765-773. Available from: https://academic.oup.com/ajcn/article/71/3/765/4729175.
- Chertow GM, Ling J, Lew NL, Lazarus JM, Lowrie EG. The association of intradialytic parenteral nutrition administration with survival in hemodialysis patients. Am J Kidney Dis. 1994;24(6):912-920. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7985668.
- Bossola M, Scribano D, Colacicco L, Tavazzi B, Giungi S, Zuppi C, Luciani G, Tazza L. Anorexia and Plasma Levels of Free Tryptophan, Branched Chain Amino Acids, and Ghrelin in Hemodialysis Patients. J Ren Nutr. 2009;19(3):248-255. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19243975.
- Lim P-S, Lin Y, Chen M, Xu X, Shi Y, Bowry S, Canaud B. Precise Quantitative Assessment of the Clinical Performances of Two High-Flux Polysulfone Hemodialyzers in Hemodialysis: Validation of a Blood-Based Simple Kinetic Model Versus Direct Dialysis Quantification. Artif Organs. 2018;42(5):E55-E66. Available from: http://doi.wiley.com/10.1111/aor.13011.
- Kirsch AH, Lyko R, Nilsson L-G, Beck W, Amdahl M, Lechner P, Schneider A, Wanner C, Rosenkranz AR, Krieter DH. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant. 2017;32(1):165-172. Available from: https://academic.oup.com/ndt/article-lookup/doi/10.1093/ndt/gfw310.
- Umber A, Wolley MJ, Golper TA, Shaver MJ, Marshall MR. Amino acid losses during sustained low efficiency dialysis in critically ill patients with acute kidney injury. Clin Nephrol. 2014;81(2):93-99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24290407.
- Kneis C, Beck W, Boenisch O, Klefisch F, Deppisch R, Zickler D, Schindler R. Elimination of middle-sized uremic solutes with high-flux and high-cut-off membranes: a randomized in vivo study. Blood Purif. 2013;36(3-4):287-294. Available from: https://www.karger.com/Article/FullText/356224.
- Fiedler R, Neugebauer F, Ulrich C, Wienke A, Gromann C, Storr M, Böhler T, Seibert E, Girndt M. Randomized controlled pilot study of 2 weeks’ treatment with high cutoff membrane for hemodialysis patients with elevated C-reactive protein. Artif Organs. 2012;36(10):886-893. Available from: http://doi.wiley.com/10.1111/j.1525-1594.2012.01479.x.
- Schmidt JJ, Hafer C, Clajus C, Hadem J, Beutel G, Schmidt BMW, Kielstein JT. New high-cutoff dialyzer allows improved middle molecule clearance without an increase in albumin loss: a clinical crossover comparison in extended dialysis. Blood Purif. 2012;34(3-4):246-252. Available from: https://www.karger.com/Article/FullText/342631.
- Krieter DH, Lemke H-D, Wanner C. A new synthetic dialyzer with advanced permselectivity for enhanced low-molecular weight protein removal. Artif Organs. 2008;32(7):547-554. Available from: http://doi.wiley.com/10.1111/j.1525-1594.2008.00583.x.
- Tomo T, Matsuyama M, Nakata T, Kadota J-I, Toma S, Koga N, Fukui H, Arizono K, Takamiya T, Matsuyama K, et al. Effect of high fiber density ratio polysulfone dialyzer on protein removal. Blood Purif. 2008;26(4):347-353. Available from: https://www.karger.com/Article/FullText/133430.
- Nakashima A, Ogata S, Doi S, Yamahira M, Naraki S, Takasugi N, Ohmoto T, Ito T, Masaki T, Yorioka N. Performance of polysulfone membrane dialyzers and dialysate flow pattern. Clin Exp Nephrol. 2006;10(3):210-215. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17009079.
- Klingel R, Ahrenholz P, Schwarting A, Röckel A. Enhanced functional performance characteristics of a new polysulfone membrane for high-flux hemodialysis. Blood Purif. 2002;20(4):325-333. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12169840.
- Akizawa T, Kinugasa E, Sato Y, Kohjiro S, Naitoh H, Azuma M, Mizutani S, Ideura T. Development of a new cellulose triacetate membrane with a microgradient porous structure for hemodialysis. ASAIO J. 44(5):M584-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9804500.
- Towards improving HD efficiency .. HD membranes update – prof. Hesham…. Available from: https://www.slideshare.net/DrMohaM/towards-improving-hd-efficiency-hd-membranes-update-prof-hesham-elsayed.
- Ward RA. Protein-leaking membranes for hemodialysis: a new class of membranes in search of an application? J Am Soc Nephrol. 2005;16(8):2421-2430. Available from: http://www.jasn.org/cgi/doi/10.1681/ASN.2005010070.
- Tsuchida K, Minakuchi J. Albumin Loss Under the Use of the High-Performance Membrane. In: Contributions to Nephrology. ; 2011:76-83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21865779.
- Belmouaz M, Diolez J, Bauwens M, Duthe F, Ecotiere L, Desport E, Bridoux F. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle-sized molecules. Clin Nephrol. August 2017. Available from: https://www.dustri.com/index.php?id=8&artId=16164&doi=10.5414/CN109133.
- Wolley M, Jardine M, Hutchison CA. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin J Am Soc Nephrol. 2018;13(5):805-814. Available from: http://cjasn.asnjournals.org/lookup/doi/10.2215/CJN.10110917.
- Tsuchida K, Minakuchi J. Albumin Loss Under the Use of the High-Performance Membrane. In: High-Performance Membrane Dialyzers. ; 2011:76-83. Available from: https://www.karger.com/Article/FullText/328957.
- Krishnasamy R, Hawley CM, Jardine MJ, Roberts MA, Cho Y, Wong M, Heath A, Nelson CL, Sen S, Mount PF, et al. A tRial Evaluating Mid Cut-Off Value Membrane Clearance of Albumin and Light Chains in HemoDialysis Patients: A Safety Device Study. Blood Purif. 2020.
- Cozzolino M, Magagnoli L, Ciceri P, Conte F, Galassi A. Effects of a medium cut-off (Theranova V R ) dialyser on haemodialysis patients: a prospective, cross-over study. Available from: https://academic.oup.com/ckj/advance-article-abstract/doi/10.1093/ckj/sfz155/5621471.
- Zickler D, Schindler R, Willy K, Martus P, Pawlak M, Storr M, Hulko M, Boehler T, Glomb MA, Liehr K, et al. Medium Cut-Off (MCO) Membranes Reduce Inflammation in Chronic Dialysis Patients-A Randomized Controlled Clinical Trial. Eller K, ed. PLoS One. 2017;12(1):e0169024. Available from: http://dx.plos.org/10.1371/journal.pone.0169024.
- Lim J-H, Park Y, Yook J-M, Choi S-Y, Jung H-Y, Choi J-Y, Park S-H, Kim C-D, Kim Y-L, Cho J-H. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. 2020.
- Bunch A, Sanchez R, Nilsson L, Bernardo AA, Vesga JI, Ardila F, Guerrero IM, Sanabria RM, Rivera AS, Arias A, et al. Medium Cut‐off Dialyzers in a Large Population of Hemodialysis Patients in Colombia: COREXH Registry . Ther Apher Dial. April 2020.
- 431 Evaluation of Biomarkers in Dhronic Hemodialysis (HD) Patients Dialyzed With Optiflux High-Flux Dialyzers. Am J Kidney Dis. 2020;75(4):662. Available from: http://www.sciencedirect.com/science/article/pii/S0272638620304765.
- Li Y, Wang Y, Lv J, Wang M. Clinical outcomes for maintenance hemodialysis patients using a high-flux (FX60) dialyzer. Ren Fail. 2013;35(9):1240-1245.
- Ward RA. Protein-Leaking Membranes for Hemodialysis: A New Class of Membranes in Search of an Application? J Am Soc Nephrol. 2005;16(8):2421-2430. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15975998.
- Galli F, Benedetti S, Floridi A, Canestrari F, Piroddi M, Buoncristiani E, Buoncristiani U. Glycoxidation and inflammatory markers in patients on treatment with PMMA-based protein-leaking dialyzers. Kidney Int. 2005;67(2):750-759. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0085253815505139.
- Kawanishi H, Mineshima M, Takesawa S, Masakane I, Minakuchi J, Akisawa T, Saito A. New Standard of Dialysis Fluid and Classification of Dialysis Membrane.; 2005.
- Osicka TM, Houlihan CA, Chan JG, Jerums G, Comper WD. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes. 2000;49(9):1579-1584. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10969843.
- Himmelfarb J, McMonagle E. Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int. 2001;60(1):358-363. Available from: http://linkinghub.elsevier.com/retrieve/pii/S008525381547855X.
- Ward RA, Brinkley KA. A proteomic analysis of proteins removed by ultrafiltration during extracorporeal renal replacement therapy. Contrib Nephrol. 2004;141:280-291. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14650239.
- Kaysen GA, Rathore V, Shearer GC, Depner TA. Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int. 1995;48(2):510-516. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7564120.
- Kaysen GA. Biological basis of hypoalbuminemia in ESRD. J Am Soc Nephrol. 1998;9(12):2368-2376. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9848794.
- Niwa T, Yazawa T, Kodama T, Uehara Y, Maeda K, Yamada K. Efficient removal of albumin-bound furancarboxylic acid, an inhibitor of erythropoiesis, by continuous ambulatory peritoneal dialysis. Nephron. 1990;56(3):241-245. Available from: https://www.karger.com/Article/FullText/186147.
- Miyata T, Ueda Y, Shinzato T, Iida Y, Tanaka S, Kurokawa K, van Ypersele de Strihou C, Maeda K. Accumulation of albumin-linked and free-form pentosidine in the circulation of uremic patients with end-stage renal failure: renal implications in the pathophysiology of pentosidine. J Am Soc Nephrol. 1996;7(8):1198-1206. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8866413.
- De Smet R, Van Kaer J, Van Vlem B, De Cubber A, Brunet P, Lameire N, Vanholder R. Toxicity of free p-cresol: a prospective and cross-sectional analysis. Clin Chem. 2003;49(3):470-478. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12600960.
- Nagai K, Tsuchida K, Ishihara N, Minagawa N, Ichien G, Yamada S, Hirose D, Michiwaki H, Kanayama H-O, Doi T, et al. Implications of Albumin Leakage for Survival in Maintenance Hemodialysis Patients: A 7-year Observational Study. Ther Apher Dial Off peer-reviewed J Int Soc Apher Japanese Soc Apher Japanese Soc Dial Ther. 2017;21(4):378-386.
P/N 104405-01 Rev A 08/2020
