Hemodialysis
Hemodialysis (HD) is the most commonly used extracorporeal clearance modality. HD attempts to replace the natural kidney function through the elimination of excess toxins and fluids that can accumulate in patients with end-stage renal disease (ESRD). HD may be utilized as an intermittent (thrice-weekly) or daily (5-7 days/week) therapy, depending on the needs and preferences of the patient. While both frequent and intermittent therapies allow for adequate solute and fluid removal, the schedules differ with respect to rates of solute equilibration and hemodynamic fluctuations. HD may also be performed both in-center and at home. Regardless of the setting or frequency of therapy, all HD sessions are performed using three main components: a dialyzer, dialysate fluid (“cleansing fluid”), and a blood delivery system.
Components of Hemodialysis 1,2
 The dialyzer is a plastic chamber composed of bundles of capillary tubes. Dialyzer membranes are comprised of one of several materials: cellulose, substituted cellulose, cellulosynthetic, and synthetic membranes. Cellulose membranes have hydroxyl groups on their surface that render them bioincompatible, meaning they can activate the compliment system. Due to their bioincompatibility, the use of cellulose membranes has decreased over the past two decades3,4. Synthetic membranes such as polysulfone, polymethylmethacrylate, and polyacrylonitrile are more biocompatible than cellulosynthetic because they lack the hydroxyl groups on their surface5.
The dialyzer is a plastic chamber composed of bundles of capillary tubes. Dialyzer membranes are comprised of one of several materials: cellulose, substituted cellulose, cellulosynthetic, and synthetic membranes. Cellulose membranes have hydroxyl groups on their surface that render them bioincompatible, meaning they can activate the compliment system. Due to their bioincompatibility, the use of cellulose membranes has decreased over the past two decades3,4. Synthetic membranes such as polysulfone, polymethylmethacrylate, and polyacrylonitrile are more biocompatible than cellulosynthetic because they lack the hydroxyl groups on their surface5.
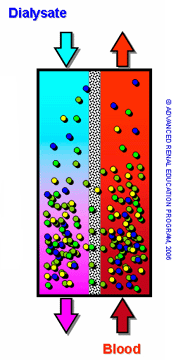
Dialysate fluid usually contains electrolytes such as potassium, calcium, sodium, magnesium, chloride, bicarbonate, and carbohydrates such as glucose. The extracorporeal circuit consisting of the dialysis machine and access (fistula, graft, or catheter) constitutes the blood delivery system for dialysis. Blood is pumped through capillary tubes, while dialysate circulates on the outside of the tubes. The blood pump moves blood from access (fistula, graft, or catheter) to the dialysis machine and back to patient while dialysate is flowing in the opposite direction. The removal of toxins and other solutes primarily depends on the diffusion rates.
Mechanisms of Solute and Fluid Removal in HD
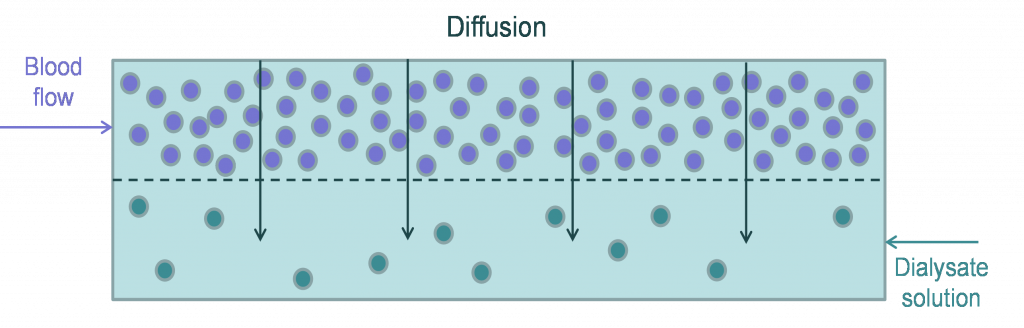
Solute removal in HD primarily occurs through a process known as diffusion where molecules passively flow from an area of high concentration (i.e., blood) to an area of low concentration (i.e., dialysate fluid) 6. Convective removal removes excess plasma water by ultrafiltration, due to the pressure gradient (via hydrostatic and osmotic pressures), and helps remove larger molecular‐weight solutes by the process of solvent drag.
Diffusive removal in HD
During the HD procedure, blood and dialysate flow through the dialyzer in a countercurrent manner, thus preventing concentration equilibration and maximizing solute transfer. This ensures that the blood is constantly exposed to fresh dialysate fluid, enabling passive diffusion of toxins from the blood to the dialysate through the semipermeable membrane of the dialyzer.
Diffusion is a passive movement of solutes “down” their concentration gradients. Solutes move from a region of higher solute concentration to a region of lower solute concentration. In terms of HD, simple passive diffusion enables clearance of endogenous waste and other solutes such as toxins from blood into the dialysate. Diffusion and clearance of solutes depend on factors such as blood and dialysate flow rates, the dialyzer membrane composition, and solute characteristics. For instance, higher blood flow rate increases clearance by increasing the amount of blood being cleared per minute7. In addition, faster dialysate flow rate causes increased removal of solutes from the dialysate fluid resulting in a higher concentration gradient and a higher clearance rate8–10. Of note, the impact of blood flow is limited to small molecular weight (MW) molecules and is further complicated by patient specific access issues7,11. The blood flow rates may range from 250–500 mL/min while a typical dialysate flow rate is 500-800 mL/min.
Diffusion of solutes also depends on the specific characteristics of the dialyzer membrane, which can affect the ability of the dialyzer to remove solutes. Dialyzer membranes can be classified as low-flux, high-efficiency, or high-flux. Low-flux dialyzers have small pores that limit clearance to small MW molecules (≤500 Daltons), such as urea and creatinine, and have a lower water permeability. High-efficiency dialyzers have large surface areas with small and large pores; therefore, they have a greater ability to remove water, small molecules such as urea, and potentially larger MW molecules such as 2-microglobulin (variable clearance) 12. High-flux membranes have larger pores that enable the removal of higher MW substances, such as β2-microglobulin (>20 ml/min) (1000 to >15,000 Daltons) and have a higher water permeability12 . High-efficiency and high-flux membranes provide greater clearance of both low and high MW substances and are more efficient, allowing for shorter treatment times, compared to low-flux membrane. Of the different types of membranes cellulose membranes are low-flux, modified or substituted cellulose membranes can be either low- or high-flux, and synthetic membranes can also be low- or high-flux.
Diffusion also is affected by characteristics of the solute, which can either increase or decrease the diffusion rate. In general, small molecules can rapidly diffuse across the semipermeable membrane, while larger molecules diffuse slowly, given that the blood and dialysate flows are constant. In most cases, uncharged solutes diffuse more rapidly than charged molecules; however, charged molecules may have increased clearance due to adsorption to synthetic membranes.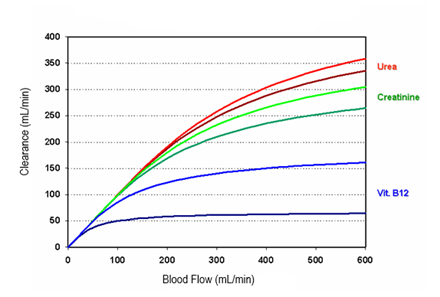
Figure 1: HD solute removal pattern. Adapted from Golper, T.A. et al.13
The figure shows the typical solute removal pattern for HD as it results from the performance of commonly used dialyzers (high flux type – bright colors, low flux type – darker colors).
Convective removal in HD
Ultrafiltration resulting in convective removal is dependent on the transmembrane pressure across the membrane (net difference of hydrostatic pressure and oncotic pressure in the blood compartment and hydrostatic pressure in the dialysate compartment). The diffusion and convection are not events that are isolated in time; however, both occur concurrently and as a result interfere with each other. The removal of solutes and fluids is enhanced via bulk flow in combination with ultrafiltration. Convection is used to augment the diffusion related transport, especially for larger molecules, given that they are able to pass through membrane pores. The hydrostatic pressure gradient across the dialysis membrane and the water permeability of a dialyzer impacts convection. While blood flow rate may vary based on access, hydrostatic pressure on the dialysate side can be manipulated to achieve desire amount of fluid removal. In addition, semipermeable membranes have different ultrafiltration coefficients (ml/min/mmHg), which affects fluid removal.
Summary
HD is primarily a diffusion‐based removal of low‐MW solutes. Excess plasma water is removed by a UF‐driven process that also helps in removing higher MW solutes that depend on convective removal. A number of factors such as, blood and dialysate flow rates, the dialyzer membrane structure and geometry can have an important influence on the efficiency of solute and fluid removal by dialysis. Additionally, the timing and frequency of dialysis also play an important role in determining patient outcomes. More frequent and/or longer dialysis not only provides a higher dose of dialysis in terms of small‐solute removal (larger number of more efficient segments) but also improves the clearance of higher MW solutes. Understanding the interplay of all the above-mentioned factors can help provide efficient dialysis to the patients and improved clinical parameters.
References:
- Sowinski KM, Churchwell MD, Decker BS. Chapter 30. Hemodialysis and Peritoneal Dialysis. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey LM, eds. Pharmacotherapy: A Pathophysiologic Approach, 9e. The McGraw-Hill Companies; 2014. Available from: accesspharmacy.mhmedical.com/content.aspx?aid=57484208.
- Liu KD, Chertow GM. Dialysis in the Treatment of Renal Failure. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education; 2018. Available from: accessmedicine.mhmedical.com/content.aspx?aid=1159155690.
- Zhang D-L, Liu J, Cui W-Y, Ji D-Y, Zhang Y, Liu W-H. Differences in bio-incompatibility among four biocompatible dialyzer membranes using in maintenance hemodialysis patients. Ren Fail. 2011;33(7):682-691. Available from: http://www.tandfonline.com/doi/full/10.3109/0886022X.2011.589943.
- John T Daugirdas, Peter G Blake TSI. Handbook of Dialysis. 5th Editio. (John T Daugirdas, Peter G Blake TSI, ed.). Wolters Kluwer Health/Inkling; 2015. Available from: https://www.inkling.com/read/daugirdas-handbook-dialysis-5/chapter-1/chapter-1-introduction.
- Bouré T, Vanholder R. Which dialyser membrane to choose? Nephrol Dial Transplant. 2004;19(2):293-296. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14736948.
- Misra M. Basic mechanisms governing solute and fluid transport in hemodialysis. Hemodial Int. 2008;12 Suppl 2:S25-8.
- Borzou SR, Gholyaf M, Zandiha M, Amini R, Goodarzi MT, Torkaman B. The effect of increasing blood flow rate on dialysis adequacy in hemodialysis patients. Saudi J Kidney Dis Transpl. 2009;20(4):639-642. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19587507.
- Azar AT. Increasing dialysate flow rate increases dialyzer urea clearance and dialysis efficiency: an in vivo study. Saudi J Kidney Dis Transpl. 2009;20(6):1023-1029. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19861865.
- Ikizler TA, Schulman G. Hemodialysis: Techniques and Prescription. Am J Kidney Dis. 2005;46(5):976-981. Available from: https://www.sciencedirect.com/science/article/pii/S0272638605010486?via%3Dihub.
- Ward RA, Idoux JW, Hamdan H, Ouseph R, Depner TA, Golper TA. Dialysate flow rate and delivered Kt/Vurea for dialyzers with enhanced dialysate flow distribution. Clin J Am Soc Nephrol. 2011;6(9):2235-2239. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21799145.
- Hassell DR, van der Sande FM, Kooman JP, Tordoir JP, Leunissen KM. Optimizing dialysis dose by increasing blood flow rate in patients with reduced vascular-access flow rate. Am J kidney Dis Off J Natl Kidney Found. 2001;38(5):948-955.
- Haroon S, Davenport A. Choosing a dialyzer: What clinicians need to know. Hemodial Int. 2018;22(S2):S65-S74. Available from: https://doi.org/10.1111/hdi.12702.
- Golper TA, Fissell R, Fissell WH, Hartle PM, Sanders ML, Schulman G. Hemodialysis: core curriculum 2014. Am J kidney Dis Off J Natl Kidney Found. 2014;63(1):153-163.
PN 102546-01 Rev B 02/2021