Management of Catheter Infections
There are two different types of catheter infections, exit-site infections and tunnel infection. Exit-site infections (ESIs) are defined as “presence of purulent drainage from the exit-site with or without erythema at the catheter-epidermal interface”(1). Tunnel infections do not often present with obvious clinical symptoms but may show signs such as “erythema, edema, or tenderness of subcutaneous pathways”. Although tunnel infections (TIs) are usually present with ESIs, they can occur independently(1). ESIs and TIs are closely related to peritonitis and remain a significant source of treatment failure and increased mortality and morbidity among peritoneal dialysis (PD) patients. Patients with a catheter infection have increased risk of catheter loss (18%), peritonitis from the same or other organisms, and overall PD technique failure(2,3).
There are many factors that could have an impact on catheter infections and loss of catheter. These include the type of catheter used, exit-site and tunnel configuration, PD exchange method or PD modality (Table 1).
| Table 1: Potential Factors Influencing Catheter Infections | |
| Type of catheter | |
|
|
|
|
| Exit and tunnel configuration | |
|
|
|
|
|
|
| PD exchange method (standard vs disconnect systems) | |
| PD modality (CAPD vs APD) | |
Types of Catheter
Several types of catheters have been designed to optimize the configuration of the subcutaneous tunnel; however, studies have not shown that any one configuration is better than another(4). In addition, whether the number of cuffs affects the clinical outcomes is also inconclusive. An analysis of the U.S. CAPD Registry data showed that single-cuff catheter placed in deep fascia had lower catheter survival rate than double-cuff and subcutaneously placed single-cuff catheters. Subcutaneously placed single-cuff catheters were also associated with higher rates of catheter removal from ESIs compared to double-cuff catheters (13% vs.7%), but lower rates of catheter removal from peritonitis compared to single-cuff catheter placed in deep fascia (14% vs. 24%)(5). A retrospective study from Canada reported lower rates of S. aureus infections in patients with double-cuff catheters who started PD between 1996 and 2000; however, the trend was not significant in those who started PD after 2000(6). Based on these reports, the ISPD guidelines state that there is no conclusive evidence to support that a certain type of catheter has better outcomes than the standard, silicone Tenckhoff catheter(1).
Exit and Tunnel Configuration
It is important to create a tunnel deep enough and away from the dermis to avoid superficial erosion of the external cuff. The recommended practice is to place the external cuff 1-2 cm from the skin opening. There is no evidence to suggest that the length of the tunnel has any impact on the incidence of ESI. A downward orientation of the catheter exit was first recommended by Tenckhoff and is considered best practice(7). Although there are no randomized studies to show the superiority of this method over a cephalad (upward or toward the head) orientation, the general opinion is that it reduces exit-site infections by facilitating drainage.
Peritoneal Dialysis Exchange Method and Modality
The use of disconnect systems has markedly reduced infectious complications of PD including ESIs and peritonitis caused by ESIs. Some studies suggested that automated peritoneal dialysis (APD)—which is a disconnect system—has been shown to be associated with fewer episodes of peritonitis than continuous ambulatory peritoneal dialysis (CAPD)(8–10). However, conflicting results have been published where no difference in the incidences of catheter related infections and peritonitis between the two modalities (11–13). The ISPD guidelines suggests that the risk of peritonitis may be similar in both modalities; therefore, the decision to use method should not be based on peritonitis risk(1). Other factors may influence the occurrence of ESIs. For instance, extruding granulation tissue or “proud flesh” can become inflamed at the exit site. If this occurs, it is often treated by simple excision or cauterization. Other primary skin or allergic conditions can also cause local inflammation and complicate exit-site care. Materials used in the manufacturing of the catheter may have an impact. The silastic material used in the manufacturing of most catheters can produce mild local inflammation that usually goes away quickly, but the Dacron cuff can cause a foreign body reaction that can last much longer. This local inflammation could leave the exit site and tunnel vulnerable to bacterial growth(14).
Causative Organisms
Per the 2011 ISPD Position statement on reducing the risks of peritonitis-related infection, Staphylococcus aureus (S.aureus) and Pseudomonas aeruginosa (P.aeruginosa) are most common pathogens that cause catheter infections, and may lead to peritonitis if not treated promptly(1–3). P.aeruginosa infections are less common compared to S.aureus, but are more difficult to eradicate and usually require longer treatment (1). Coagulase negative Staphylococci, fungi and other Gram-positive organisms account for the remaining infections. The spectrums of causative organisms in ESIs and peritonitis are depicted in figure 1 and figure 2(15).
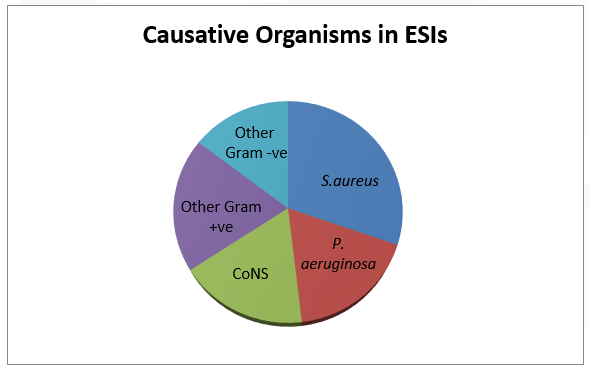 Figure 1: Organisms involved with ESIs in the U.S (2006).
Figure 1: Organisms involved with ESIs in the U.S (2006).
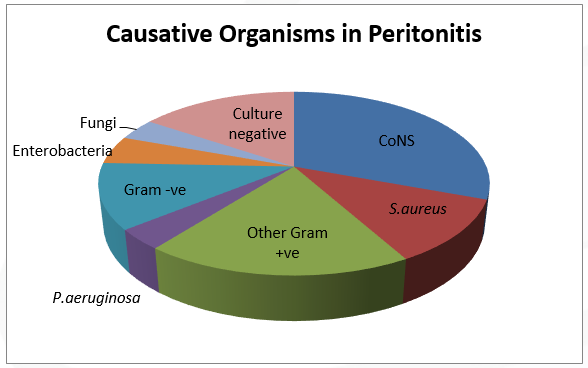 Figure 2: Organisms involved in peritonitis in PD patients in the U.S (2006)
Figure 2: Organisms involved in peritonitis in PD patients in the U.S (2006)
Treatment
Empiric antibiotic therapy should be started immediately for tunnel and exit-site infections, pending culture results, and should follow recommended best practice guidelines such as ISPD guidelines , and should cover S. aureus (16). Complete removal of the superficial cuff can be performed when antibiotics do not resolve an infection. However, it must be noted that peritonitis with the same organism occurs in 50% of patients who undergo cuff shaving for S. aureus and may result in eventual removal of the catheter. Additionally, topical treatment can be used as an adjunct to systemic antibiotics in the treatment of exit infections or initial therapy for low-grade infection or equivocal sites. Topical antibiotic therapy is not appropriate for acute and chronic exit infections. Topical antibiotic creams but not ointments should be used with polyurethane catheters such as the Cruz catheter; some ointment formulations contain alcohol as an inactive ingredient, which can degrade the catheter and cause it to crack(17). Cauterization of exuberant granulation tissue in the sinus may be necessary. Systemic antibiotics may be needed in cases unresponsive to topical therapy.
Hypertonic saline dressings may also be beneficial in early exit-site infection. Such topical treatments include application of soaks to the exit 2-4 times per day, as well as the application of dry heat. Soaking solutions include 0.9% saline, sodium hypochlorite, dilute hydrogen peroxide, and povidone iodine(18). However, there are no controlled studies assessing the effectiveness of these treatments.
Care of the infected exit site includes daily or twice daily exit-site care. The forceful removal the crusts or scabs is not recommended. Their removal can be enhanced by gradual softening with hydrogen peroxide, saline, soap and water, or exit soaks. Sterile dressings are used to absorb drainage, reduce exposure to microorganisms and protect the site from trauma. Notably, a recent study that compared a dressing group to a non-dressing group showed no difference in the incidences of PD catheter-related infections between the two groups. This result suggests that daily cleansing with antibacterial soap and use of prophylactic cream without dressing may be adequate to prevent PD catheter-related infections (19).
When an exit site, cuff, or tunnel infection is associated with peritonitis due to the same organism, catheter removal is considered, unless the organism is S. epidermidis. Even though the effluent may clear with antibiotic use, the culture often remains positive, and peritonitis will recur unless the source catheter is removed. The catheter is also typically removed in cases of refractory or recurrent peritonitis with exit or tunnel infections or extensive cellulitis unresponsive to antibiotics. Early catheter removal may be also be necessary if pseudomonal ESIs fail to respond to appropriate antibiotic therapy and shaving of the external cuff(20).
References
- Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye W-C, Price V, Ramalakshmi S, Szeto C-C. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int. 2011;31(6):614-630. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21880990.
- Campbell DJ, Johnson DW, Mudge DW, Gallagher MP, Craig JC. Prevention of peritoneal dialysis-related infections. Nephrol Dial Transplant. 2015;30(9):1461-1472. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25294849.
- Akoh JA. Peritoneal dialysis associated infections: An update on diagnosis and management. World J Nephrol. 2012;1(4):106-122. Available from: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3782204&tool=p….
- Eklund BH, Honkanen EO, Kala AR, Kyllönen LE. Peritoneal dialysis access: prospective randomized comparison of the Swan neck and Tenckhoff catheters. Perit Dial Int. 1995;15(8):353-356. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8785234.
- Lindblad AS, Novak JW, Nolph KD, Stablein DM, Cutler SJ. The 1987 USA National CAPD Registry report. ASAIO Trans. 1988;34(2):150-156. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3370177.
- Nessim SJ, Bargman JM, Jassal S V. Relationship between double-cuff versus single-cuff peritoneal dialysis catheters and risk of peritonitis. Nephrol Dial Transplant. 2010;25(7):2310-2314. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20103501.
- Tenckhoff H, Schechter H. A bacteriologically safe peritoneal access device. Trans Am Soc Artif Intern Organs. 1968;14:181-187. Available from: https://www.ncbi.nlm.nih.gov/pubmed/5701529.
- Brunkhorst R, Wrenger E, Krautzig S, Ehlerding G, Mahiout A, Koch KM. Clinical experience with home automated peritoneal dialysis. Kidney Int Suppl. 1994;48:S25-S30. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7700038.
- Holley JL, Bernardini J, Piraino B. Continuous cycling peritoneal dialysis is associated with lower rates of catheter infections than continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 1990;16(2):133-136. Available from: https://www.ncbi.nlm.nih.gov/pubmed/2382649.
- Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM. Automated vs continuous ambulatory peritoneal dialysis: a systematic review of randomized controlled trials. Nephrol Dial Transplant. 2007;22(10):2991-2998. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17875571.
- Rodríguez-Carmona A, Pérez Fontán M, García Falcón T, Fernández Rivera C, Valdés F. A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int. 1999;19(3):253-258. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10433162.
- Cnossen TT, Usvyat L, Kotanko P, van der Sande FM, Kooman JP, Carter M, Leunissen KML, Levin NW. Comparison of outcomes on continuous ambulatory peritoneal dialysis versus automated peritoneal dialysis: results from a USA database. Perit Dial Int. 2011;31(6):679-684. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20829519.
- Aslam N, Bernardini J, Fried L, Burr R, Piraino B. Comparison of infectious complications between incident hemodialysis and peritoneal dialysis patients. Clin J Am Soc Nephrol. 2006;1(6):1226-1233. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17699352.
- Swartz RD. Exit-site and catheter care: review of important issues. Adv Perit Dial. 1999;15:201-204. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10682102.
- Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl. 2006;(103):S55-S62. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17080112.
- Li PK-T, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye W-C, Salzer W, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20628102.
- The Mupirocin Study Group. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. Mupirocin Study Group. J Am Soc Nephrol. 1996;7(11):2403-2408. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8959632.
- Kathuria P, Twardowski ZJ, Nichols WK. Peritoneal Dialysis Access and Exit-Site Care Including Surgical Aspects. In: Khanna R, Krediet RT, eds. Nolph and Gokal’s Textbook of Peritoneal Dialysis. 3rd ed. New York: Springer Science+Business Media; 2009:371-446.
- Mushahar L, Mei LW, Yusuf WS, Sivathasan S, Kamaruddin N, Idzham NJM. Exit-Site Dressing and Infection in Peritoneal Dialysis: A Randomized Controlled Pilot Trial. Perit Dial Int. 2016;36(2):135-139. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26374836.
- Freitas C, Rodrigues A, Carvalho MJ, Cabrita A. Exit site infections: systematic microbiologic and quality control are needed. Adv Perit Dial. 2009;25:26-31. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19886313.
P/N 102494-01 Rev. A 07/2016
