Managing Hyperphosphatemia in Adult Patients with Chronic Kidney Disease: Assessment of Outcomes with Calcium-Based Phosphate Binders
Background
Hyperphosphatemia is a common clinical challenge associated with advanced renal disease, and it has been linked with increased cardiovascular mortality (1). The current standard of care for the treatment of hyperphosphatemia in patients with later stages of chronic kidney disease, including those undergoing dialysis, involves dialytic, non-pharmacologic (e.g., dietary), and/or pharmacologic interventions. A variety of phosphate-binding drugs have been studied and used in clinical practice as a means of keeping serum phosphorus levels within a normal range, including calcium-based phosphate binders (CBPBs) and non-CBPBs. While CBPBs continue to be used widely in clinical practice, the literature shows ongoing concerns regarding the potential of these agents to create a positive calcium balance, which may, in turn, lead to vascular calcification (2)–(5). In addition, the literature raises other concerns, including patient mortality, with the use of CBPBs (6)–(8). Different classes of phosphate binder therapy, including CBPBs, may increase the substantial pill burden facing many patients with advanced chronic kidney disease, leading to suboptimal rates of treatment adherence (9),(10).
In 2017, the Kidney Disease Improving Global Outcomes (KDIGO) foundation updated its Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline, including several key revisions regarding the use of phosphate binders in the management of hyperphosphatemia for patients with advanced renal disease. Among these revisions is the suggestion that in adult patients with CKD G3a-G5D (having a glomerular filtration rate < 60 mL/min/1.73 m2), the use of calcium-based phosphate binders should be restricted (11). This update broadens the scope of patients in whom calcium-based phosphate binders should be restricted, as previous guidelines only made this recommendation for patients with persistent/recurrent hypercalcemia, arterial calcification, adynamic bone disease , and/or persistently low serum parathyroid hormone levels (12). The level of supporting evidence for this revised guideline suggestion was characterized as moderate-quality, i.e., “the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different” (11). Subsequent investigations have sought to further clarify the association between calcium-based phosphate binders and different patient outcomes.
Given the lack of standardization regarding phosphate binder selection for adult patients seen in clinical practice, it is imperative that clinicians clearly understand available evidence regarding use of calcium-based phosphate binders on clinical outcomes.
Mortality
Several studies have provided additional data which helped shape the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guideline Update. Two studies evaluating calcium balance in patients with CKD (13),(14) and three additional randomized controlled trials (RCTs) (15)–(17) were instrumental in leading to the expansion of recommendations that restrict the use of CBPBs in adult patients with CKD G3a-G5D.
Two of the RCTs compared mortality outcomes with CBPB therapy and non-CBPB therapy. The first of these studies, conducted by Di Iorio et al., compared mortality outcomes in 212 patients with stage 3-4 CKD between those randomized to sevelamer (a non-CBPB therapy) and those randomized to calcium carbonate therapy over three years. In this study, the all-cause mortality rate was significantly greater among patients receiving calcium carbonate (P <.05) (17). A second randomized study, also conducted by Di Iorio et al., compared mortality outcomes in 466 incident hemodialysis patients between those randomized to sevelamer therapy and those receiving calcium carbonate therapy over two years. Patients treated with sevelamer had a lower rate of cardiovascular mortality due to cardiac arrhythmias than those treated with calcium carbonate (HR 0.06, CI 0.01-0.25; P <.001) (16). Better survival outcomes were observed with respect to all-cause cardiovascular mortality and all-cause mortality in patients treated with sevelamer, but not non-cardiovascular mortality. The authors were not able to specify whether the improvement in outcomes was attributable to improved serum phosphorus control or reduction in calcium load (16).
In its assessment of the 2017 KDIGO CKD-MBD Guideline update, which was also published in 2017, a KDOQI work group questioned the strength of recommendation regarding calcium-based phosphate binders given by the KDIGO guidelines for adult patients. This KDOQI CKD-MBD work group, convened by the National Kidney Foundation, cited insufficient adequately powered trials examining mortality, the homogeneous nature of the patient populations, and a need for more definitive data that could be applied to American patients with CKD (18). Even so, most of the KDOQI work group supported limiting the use of CBPB therapy when possible, given the efficacy of non-CBPB agents (18).
Since 2016, multiple meta-analyses and a Cochrane Database review have also evaluated the relative effect of different phosphate binders on all-cause and cardiovascular mortality (7),(19)–(22). Many of these analyses have suggested that non calcium-based phosphate binders significantly decrease all-cause mortality among adult patients with CKD relative to calcium-based phosphate binders. Mortality comparisons from these studies are summarized in Table 1 below:
Table 1: Select Meta-Analyses Addressing Mortality Associated with Phosphate Binder Therapy
| Author | Total Number of Patients Included in Meta-Analysis | Total Number of Studies in Meta-Analysis | All-Cause Mortality | Cardiovascular Mortality |
| Palmer et al. (19) | 12,562 | 77
(62 in dialysis population) |
|
|
| Sekercioglu et al. (20) | 8,335 | 28
(21 in dialysis population) |
|
|
| Patel et al. (7) | 4,770 | 25
(22 in dialysis population) |
|
|
| Habbous et al. (21) | 8,829 | 51
(43 in dialysis population) |
|
|
| Ruospo et al. (22) | 13,744 | 104
(82 in dialysis population) |
|
|
Of note, many of these meta-analyses have included heterogeneous groups of studies, where the inclusion or exclusion of one trial may alter the significance of the results (23). Furthermore, studies incorporated different types and numbers of CBPBs. Different CBPBs have been associated with different degrees of calcium absorption in short-term studies (24).
Not all studies have demonstrated better all-cause mortality outcomes for patients with non-CBPB therapy. An observational cohort study conducted by Spoendlin et al. compared all-cause mortality and a range of cardiovascular events for hemodialysis patients ≥65 years of age who received either sevelamer or calcium acetate (25). The study analysis included 2639 patients initiating treatment with sevelamer and 2065 patients initiating calcium acetate. In this study, there was no significant difference in the risk of fatal or nonfatal cardiovascular events or all-cause mortality between patients who received sevelamer and those who received calcium acetate (25). An HR of 0.96 (95% CI, 0.84-1.10) for cardiovascular events was observed. Strengths of the study included its large cohort, good covariate balance, and consistent results between as-treated and ITT follow-up models (25). Limitations include the exclusion of patients who used phosphate binders before the initiation of hemodialysis, creating a possible selection bias. A data lag for USRDS data led to the omission of iron-based phosphate binders in the analysis. The observational nature of this study limits any definitive establishment of a causal relationship (25).
The randomized Dialysis Clinical Outcomes Revisited trial, which compared all-cause and cause-specific mortality outcomes for hemodialysis patients receiving either sevelamer or CBPB therapy, showed no significant differences in mortality outcomes in the overall population with respect to phosphate binder treatment (26). However, among patients ≥65 years of age, the all-cause mortality rate was lower among patients in the sevelamer group compared with that in the CBPB group (18.2/100 patient years versus 23.4/100 patient years; HR 0.77; 95% CI 0.91-1.53) (26). While most studies have suggested lower mortality rates with the use of non-CBPB therapy compared with CBPB agents, additional long-term RCT investigations may help to clarify this relationship in different patient cohorts.
Vascular Calcification
The challenge of vascular calcification, which may contribute to increased morbidity and mortality in patients with renal disease, may also be influenced by phosphate binder selection. Patients with chronic kidney disease have a greater incidence and progression of vascular calcification (27), and data pertaining to the association of calcium-based phosphate binder therapy and the progression of vascular calcification continue to accumulate, both through randomized trials and meta-analyses. Among the studies used to justify recent KDIGO guideline changes are 2 studies on calcium balance in patients with CKD. One randomized, placebo-controlled crossover study conducted by Hill et al. evaluated calcium kinetics in patients with stage 3 or stage 4 CKD and found that calcium carbonate supplementation resulted in a positive calcium balance within 3 weeks, with suggestion of calcium deposition in soft tissues (28). Those patients who received placebo maintained a neutral calcium status (28). Another study conducted by Spiegel et al. evaluated calcium balance in patients with stage 3-4 CKD, and found that high levels of calcium intake result in a positive calcium balance (14).
A typical daily dose of calcium acetate for the treatment of hyperphosphatemia in patients with ESRD would necessitate ingestion of more than 1500 mg of elemental calcium each day; this exceeds the maximum recommended daily dose (1500 mg) of elemental calcium from phosphate binders according to 2003 KDOQI guidelines for patients with ESRD (29). A recent post-hoc analysis of 752 patients with ESRD found that nearly half of the 551 patients with calcium acetate data had an elemental calcium intake ≥ 1500 mg/day, and 58.2% of the 201 patients with calcium carbonate dose data had an elemental calcium intake ≥ 1500 mg/day (30). These patients may be susceptible to complications such as adynamic bone disease and vascular calcification (30).
With respect to comparative vascular calcification studies that have influenced recent guidelines, a study conducted by Block et al. randomized 148 patients with CKD to receive calcium acetate, lanthanum, sevelamer, or placebo (15). Treatment with phosphate binders was associated with increases in vascular calcification relative to placebo (Figure), both in the coronary arteries (18.1% versus 0.6%, P = .05) and abdominal aorta (15.4% versus 3.4%, P = .03), an effect most pronounced in patients receiving calcium acetate (15).
Figure: Percent Change in Median Annualized Total Coronary Artery and Abdominal Aorta Calcium Volume Scores (redrawn from Block et al.) (15)
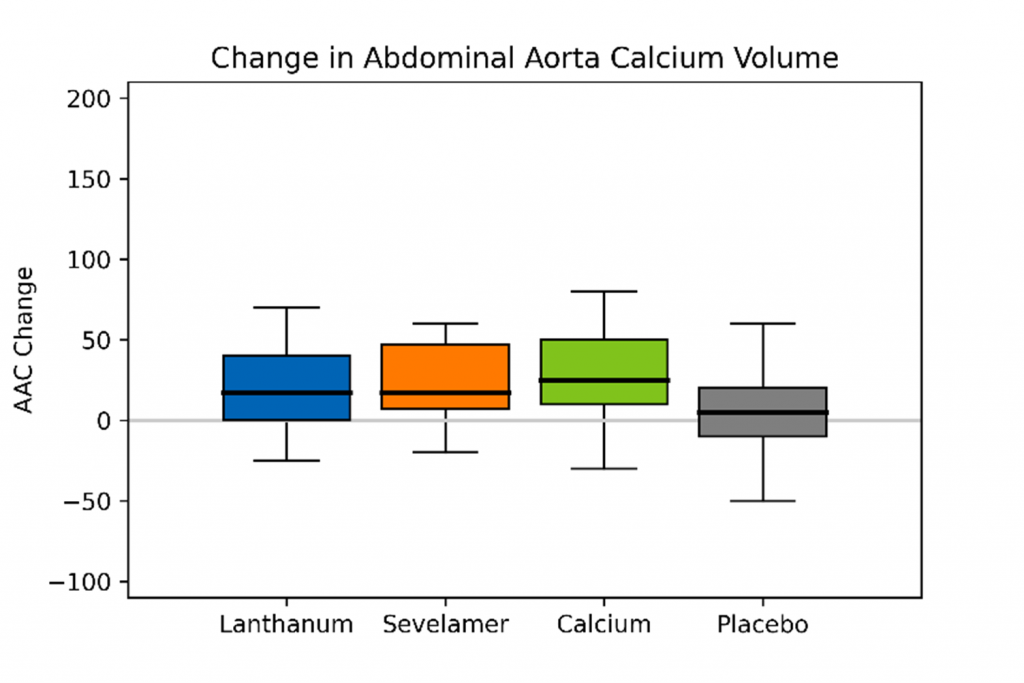
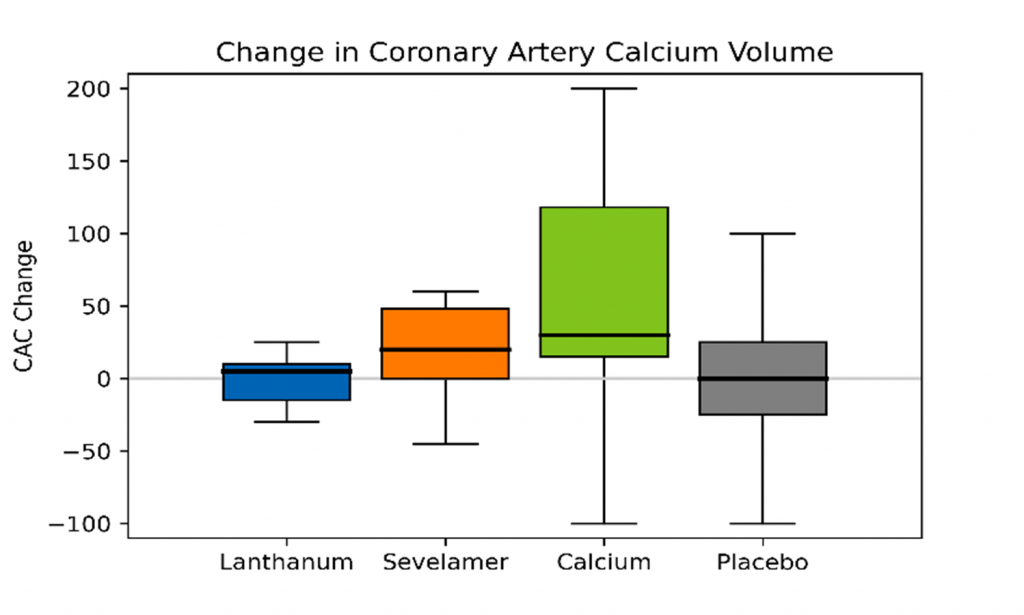
In a study conducted by Di Iorio et al., 212 patients with stage 3-4 CKD were randomized to receive either calcium carbonate or sevelamer (17). Coronary artery calcification (CAC) scores were evaluated at 6-month intervals for a period of two years. At baseline, the sevelamer group had a greater percentage of calcified patients (62.6% versus 47.6%; P = .02). Over the course of the 2-year observation period, new onset of CAC was observed in 5 patients treated with sevelamer and 45 patients treated with calcium carbonate (17). For patients who had a positive CAC score at baseline, regression of this score was observed in 24 patients treated with sevelamer and only 2 patients treated with calcium carbonate (17).
Other studies have also helped to shed light on the impact of phosphate binders on the progression of vascular calcification. The Treat to Goal (TTG) trial of 200 hemodialysis patients evaluated coronary artery and aortic calcification scores, randomizing patients to be treated with sevelamer or calcium-based phosphate binders over one year (31). In the final analysis, the median absolute calcium scores were significantly greater among patients treated with CBPBs, but not among patients treated with sevelamer (31). This was observed with both coronary artery scores (36.6 versus 0, P =.03) and aorta scores (75.1 versus 0, P = .01) (31). In addition, the median increase in coronary artery and aortic calcification scores was significantly greater with CBPBs than with sevelamer.
The role of phosphate binders in influencing progression of vascular calcification in patients new to hemodialysis has also been explored. The randomized Renagel in New Dialysis (RIND) study of 129 patients who received either sevelamer or CBPB therapy reported that increases in CAC scores were more rapid and severe among patients treated with CBPBs (P = .056 at 12 months, P = .01 at 18 months) (32).
Not all studies have demonstrated better CAC scores with the use of non-CBPB therapy for patients with CKD. In the Calcium Acetate Renagel Evaluation-2 (CARE-2) study, 203 patients on hemodialysis were randomized to receive calcium acetate or sevelamer (33). The progression of CAC with intensive lowering of LDL-C levels was similar in both groups, with a geometric mean increase of 35% for the calcium acetate group and 39% for the sevelamer group (33). The covariate-adjusted calcium acetate-sevelamer ratio was 0.994 (95% CI 0.851-1.161) (33).The difference in outcomes between CARE-2 and RIND/TTG may be accounted for by an increased prevalence of diabetic nephropathy and smoking in the CARE-2 patient cohort (34).
A recent meta-analysis of randomized controlled trials evaluated the effects of CBPBs and sevelamer on vascular calcification. In 6 of 9 studies, greater and/or more rapid increases in coronary artery calcification were reported among patients treated with CBPBs compared with those treated with sevelamer (7). No differences between the two cohorts were reported in the other three studies. This variation in results is consistent with a recent Cochrane database review, which found that for patients with CKD G2-G5, sevelamer was found to have “uncertain or inestimable effects” on coronary artery calcification relative to CBPB therapy (22). In the Cochrane review, lanthanum was also determined to have uncertain effects on CAC when compared with CBPB therapy (22). While some studies have pointed to the role of non-CBPB therapy in attenuating the rate of CAC progression, particularly among those patients with pre-existing CAC, some questions remain regarding the absolute magnitude of clinical benefit derived from different classes of phosphate binders with respect to CAC modification.
Pill Burden
Lack of adherence to phosphate binder therapy remains an ongoing challenge for patients with renal disease who may benefit from the use of these therapies. Greater pill burden has been associated with a reduction in treatment adherence, and different phosphate binders have been shown to have different pill burdens based on their relative phosphate binding efficacy (35). One study of 233 chronic dialysis patients reported that the median overall number of pills taken daily was 19, with phosphate binders accounting for nearly half the total pill burden of all different medication classes (49 ± 19%) (36). Patients taking calcium-based phosphate binders as monotherapy had a median pill burden of 9, and those who used combination therapy had a median phosphate binder burden of 13 pills daily (36). This is of clinical relevance, as a study conducted by Wang et al. suggested that greater phosphate binder pill burden may reduce the likelihood of achieving serum phosphorus goals (9). Studies have shown that some non-CBPBs may present a lower pill burden than calcium-based phosphate binders (37)–(39).
The challenge of pill burden may affect treatment adherence, as shown by an analysis of the Dialysis Outcomes and Practice Patterns Study, which reported that less than half of American dialysis patients took their entire prescription of phosphate binders during the previous month (10). Another study by Wang et al. reported phosphate binder adherence as a function of a medication possession ratio, i.e., indicating the amount of time patients had an adequate medication supply to take the medication as prescribed (9). The MPR in this study was 40-50%, similar to the level of adherence reported in the DOPPS assessment. Indeed, the range of PB nonadherence has been reported from 22 to 74%, depending on patient population and measurement method (9). In addition, pill burden associated with phosphate binders may adversely influence patient appetite and nutritional status (40). Clinical evidence has shown that some non-CBPB agents may have the potential to lower pill burden for patients on CBPB therapy and improve adherence to therapy (39),(41). Tolerability and avoidance of side effects particular to each phosphate binder type also need to be considered when selecting phosphate binder therapy, as these factors also contribute to the “burden” of medication.
Summary
Several publications have argued for the preferential use of non-CBPBs in adult patients with CKD stage 5D. While CBPBs may provide effective phosphate control and are less expensive, the literature shows that they are also associated with a greater risk of developing hypercalcemia and related sequelae. Given the current level of evidence, it would certainly seem appropriate in circumstances where it is practicable and non-CBPB agents are available to follow the recommendations of current KDIGO guidelines and limit the use of CBPBs in clinical practice for adult patients.
References:
- Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607-17. www.ncbi.nlm.nih.gov/pubmed/9531176
- Negri AL, Ureña Torres PA. Iron-based phosphate binders: do they offer advantages over currently available phosphate binders? Clin Kidney J. 2015;8(2):161-7. www.ncbi.nlm.nih.gov/pubmed/25815172
- Carfagna F, Del Vecchio L, Pontoriero G, Locatelli F. Current and potential treatment options for hyperphosphatemia. Expert Opin Drug Saf. 2018;17(6):597-607.
- Locatelli F, Del Vecchio L, Violo L, Pontoriero G. Phosphate binders for the treatment of hyperphosphatemia in chronic kidney disease patients on dialysis: a comparison of safety profiles. Expert Opin Drug Saf. 2014;13(5):551-61. www.ncbi.nlm.nih.gov/pubmed/24702470
- Kalaitzidis RG, Elisaf MS. Hyperphosphatemia and phosphate binders: Effectiveness and safety. Curr Med Res Opin. 2014;30(1):109-112.
- Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, Lok CE, Fitchett D, Tsuyuki RT. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet (London, England). 2013;382(9900):1268-77. www.ncbi.nlm.nih.gov/pubmed/23870817
- Patel L, Bernard LM, Elder GJ. Sevelamer Versus Calcium-Based Binders for Treatment of Hyperphosphatemia in CKD: A Meta-Analysis of Randomized Controlled Trials. Clin J Am Soc Nephrol. 2016;11(2):232-44. www.ncbi.nlm.nih.gov/pubmed/26668024
- Elder GJ, Center J. The role of calcium and non calcium-based phosphate binders in chronic kidney disease. Nephrology. 2017;22:42-46.
- Wang S, Alfieri T, Ramakrishnan K, Braunhofer P, Newsome BA. Serum phosphorus levels and pill burden are inversely associated with adherence in patients on hemodialysis. Nephrol Dial Transplant. 2014;29(11):2092-9. www.ncbi.nlm.nih.gov/pubmed/24009281
- Fissell RB, Karaboyas A, Bieber BA, Sen A, Li Y, Lopes AA, Akiba T, Bommer J, Ethier J, Jadoul M, Pisoni RL, Robinson BM, Tentori F. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: Findings from the DOPPS. Hemodial Int. 2015;20(1):38-49. www.ncbi.nlm.nih.gov/pubmed/25975222
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1-59. www.ncbi.nlm.nih.gov/pubmed/30675420
- Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017;92(1):26-36. www.ncbi.nlm.nih.gov/pubmed/28646995
- Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int. 2013;83(5):959-966. www.ncbi.nlm.nih.gov/pubmed/23254903
- Spiegel DM, Brady K. Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets. Kidney Int. 2012;81(11):1116-1122. www.ncbi.nlm.nih.gov/pubmed/22297674
- Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, Allison MA, Asplin J, Smits G, Hoofnagle AN, Kooienga L, Thadhani R, Mannstadt M, Wolf M, Chertow GM. Effects of Phosphate Binders in Moderate CKD. J Am Soc Nephrol. 2012;23(8):1407-1415. www.ncbi.nlm.nih.gov/pubmed/22822075
- Di Iorio B, Molony D, Bell C, Cucciniello E, Bellizzi V, Russo D, Bellasi A, INDEPENDENT Study Investigators. Sevelamer versus calcium carbonate in incident hemodialysis patients: results of an open-label 24-month randomized clinical trial. Am J Kidney Dis. 2013;62(4):771-8. www.ncbi.nlm.nih.gov/pubmed/23684755
- Di Iorio B, Bellasi A, Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7(3):487-93. www.ncbi.nlm.nih.gov/pubmed/22241819
- Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H. KDOQI US Commentary on the 2017 KDIGO Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737-751. www.ncbi.nlm.nih.gov/pubmed/28941764
- Palmer SC, Gardner S, Tonelli M, Mavridis D, Johnson DW, Craig JC, French R, Ruospo M, Strippoli GFM. Phosphate-Binding Agents in Adults With CKD: A Network Meta-analysis of Randomized Trials. Am J Kidney Dis. 2016;68(5):691-702.
- Sekercioglu N, Thabane L, Díaz Martínez JP, Nesrallah G, Longo CJ, Busse JW, Akhtar-Danesh N, Agarwal A, Al-Khalifah R, Iorio A, Guyatt GH. Comparative Effectiveness of Phosphate Binders in Patients with Chronic Kidney Disease: A Systematic Review and Network Meta-Analysis. Barretti P, ed. PLoS One. 2016;11(6):e0156891. www.ncbi.nlm.nih.gov/pubmed/27276077
- Habbous S, Przech S, Acedillo R, Sarma S, Garg AX, Martin J. The efficacy and safety of sevelamer and lanthanum versus calcium-containing and iron-based binders in treating hyperphosphatemia in patients with chronic kidney disease: A systematic review and meta-analysis. Nephrol Dial Transplant. 2017;32(1):111-125.
- Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, Strippoli GF. Phosphate binders for preventing and treating chronic kidney disease‐mineral and bone disorder (CKD‐MBD). Cochrane Database Syst Rev. 2018;2018(8):CD006023. www.ncbi.nlm.nih.gov/pubmed/30132304
- Peter WL St., Wazny LD, Weinhandl E, Cardone KE, Hudson JQ. A Review of Phosphate Binders in Chronic Kidney Disease: Incremental Progress or Just Higher Costs? Drugs. 2017;77(11):1155-1186. www.ncbi.nlm.nih.gov/pubmed/28584909
- Emmett M. A comparison of calcium-based phosphorus binders for patients with chronic kidney disease. Dial Transplant. 2006;35(5):284-293, 336.
- Spoendlin J, Paik JM, Tsacogianis T, Kim SC, Schneeweiss S, Desai RJ. Cardiovascular Outcomes of Calcium-Free vs Calcium-Based Phosphate Binders in Patients 65 Years or Older With End-stage Renal Disease Requiring Hemodialysis. JAMA Intern Med. 2019. www.ncbi.nlm.nih.gov/pubmed/31058913
- Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, Ling BN, Chasan-Taber S, Dillon MA, Blair AT, Burke SK. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72(9):1130-7. www.ncbi.nlm.nih.gov/pubmed/17728707
- Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A AV, Russo D, Miranda I, Ruocco C, Battaglia Y, Buonanno E, Manzi S, Russo L, Scafarto A, Andreucci VE. The progression of coronary artery calcification in predialysis patients on calcium carbonate or sevelamer. Kidney Int. 2007;72(10):1255-61. www.ncbi.nlm.nih.gov/pubmed/17805238
- Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3–4 chronic kidney disease. Kidney Int. 2013;83(5):959-966. www.ncbi.nlm.nih.gov/pubmed/23254903
- National Kidney Foundation, Kasiske BL, Co-Editors Blanche Chavers M, Mark Rosenberg M, Robert Foley M, Minneapolis MM, Suzanne Swan M, Cattran D, Ricardo Correa-Rotter C, City M, Jonathan Craig M, Sydney Mbc, Paul de Jong A, Golper T, Donald Hricik T, Hi Bahl Lee O, Adeera Levin K, Philip Kam Tao Li C, Kong H, et al. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Natl Kidney Found. 2003.
- Wilson RJ, Copley JB. Elemental calcium intake associated with calcium acetate/calcium carbonate in the treatment of hyperphosphatemia. Drugs Context. 2017;6.
- Chertow GM, Burke SK, Raggi P, for the Treat to Goal Working Group. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245-252. www.ncbi.nlm.nih.gov/pubmed/12081584
- Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815-1824. www.ncbi.nlm.nih.gov/pubmed/16164659
- Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M, CARE-2 Investigators. A 1-Year Randomized Trial of Calcium Acetate Versus Sevelamer on Progression of Coronary Artery Calcification in Hemodialysis Patients With Comparable Lipid Control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) Study. Am J Kidney Dis. 2008;51(6):952-965. www.ncbi.nlm.nih.gov/pubmed/18423809
- Floege J. Calcium-containing phosphate binders in dialysis patients with cardiovascular calcifications: Should we CARE-2 avoid them? Nephrol Dial Transplant. 2008;23(10):3050-3052.
- Gutekunst L. An Update on Phosphate Binders: A Dietitian’s Perspective. J Ren Nutr. 2016;26(4):209-218. www.ncbi.nlm.nih.gov/pubmed/26920090
- Chiu Y-W, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089-96. www.ncbi.nlm.nih.gov/pubmed/19423571
- Coyne DW, Ficociello LH, Parameswaran V, Anderson L, Vemula S, Ofsthun NJ, Mullon C, Maddux FW, Kossmann RJ, Sprague SM, M. S, Sprague SM. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: A retrospective analysis of pharmacy data . Clin Nephrol. 2017;88(08):59-67. www.ncbi.nlm.nih.gov/pubmed/28587714
- Kendrick J, Parameswaran V, Ficociello LH, Ofsthun NJ, Davis S, Mullon C, Kossmann RJ, Kalantar-Zadeh K. One-Year Historical Cohort Study of the Phosphate Binder Sucroferric Oxyhydroxide in Patients on Maintenance Hemodialysis. J Ren Nutr. 2019. www.ncbi.nlm.nih.gov/pubmed/30679076
- Vemuri N, Michelis MF, Matalon A. Conversion to lanthanum carbonate monotherapy effectively controls serum phosphorus with a reduced tablet burden: A multicenter open-label study. BMC Nephrol. 2011;12(1).
- Kalantar-Zadeh K, Ficociello LH, Parameswaran V, Athienites N V, Mullon C, Kossmann RJ, Coyne DW. Changes in serum albumin and other nutritional markers when using sucroferric oxyhydroxide as phosphate binder among hemodialysis patients: a historical cohort study. BMC Nephrol. 2019;20(1):396. www.ncbi.nlm.nih.gov/pubmed/31664928
- Gray K, Ficociello LH, Hunt AE, Mullon C, Brunelli SM. Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis. 2019;12:1-8. www.ncbi.nlm.nih.gov/pubmed/30774412
PN 104287-01 Rev A 5/2020
