Diabetes and Diabetic Kidney Disease
Diabetes Mellitus
Etiology and Classification
Diabetes mellitus (DM), often referred to as diabetes, is a group of metabolic diseases characterized by high glucose levels, or hyperglycemia that result from defects in insulin secretion, action, or both (1–3). DM is the most common disorder of the endocrine system and has been classified into Type 1, Type 2 and gestational diabetes. Type I DM, previously known as juvenile or insulin-dependent diabetes, accounts for 5%-10% of the total diabetic population (1–3). In Type I DM, an autoimmune reaction destroys pancreatic beta cells which results in severe insulin deficiency or complete non-production of insulin (1–3). Individuals with Type I DM typically require exogenous insulin replacement therapy and/or transplantation of pancreas or pancreatic islet cells in order to survive. Type I DM occurs most commonly in children and young adults, although disease onset can occur at any age. Type II DM, previously known as adult-onset or non-insulin dependent diabetes, accounts for 90%-95% of the diabetic population (1–3). Type II DM is associated with insulin resistance and increased insulin levels. Over time, a gradual reduction in insulin secretion by the pancreas is often seen, leading to decreasing hyperinsulinemia in later stages (1–3). Management of Type II DM focuses on lifestyle changes in combination with pharmacologic interventions. Increased risk for Type II DM is associated with older age, obesity, family history, physical inactivity, ethnicity, and a host of other factors (1–3). Gestational diabetes is thought to be caused by insulin-interfering hormones produced during pregnancy. Although gestational diabetes usually disappears after the birth of the baby, women who have had gestational diabetes have a 40 to 60 percent chance of developing Type II DM within 5 to 10 years (1–3).
Prevalence:
In 2019, it was estimated that 463 million people worldwide had diabetes and prevalence is projected to increase to 700 million by 2045 (4). Similarly, the prevalence of diabetes in the United States has been on the rise for the past three decades. Current statistics from the 2018 Centers for Disease Control and Prevention (CDC) National Diabetes Facts Sheet estimate 34.1 million adults aged 18 years or older or 13.0% of all US adults had diabetes, with over 7 million (2.8 % of all US adults) of those cases being undiagnosed. In addition, an estimated 88 million people had prediabetes in 2018 based on their fasting glucose or HbA1c level (2). During 1999–2016, the age-adjusted prevalence of total diabetes significantly increased among adults aged 18 years or older (Figure 1). The estimates of new cases of diabetes in the year 2018 among US adults aged 18 years or older were 1.5 million or 6.9 per 1,000 persons. Compared to adults aged 18 to 44 years, incidence rates of diagnosed diabetes were higher among adults aged 45 to 64 years and those aged 65 years and older.
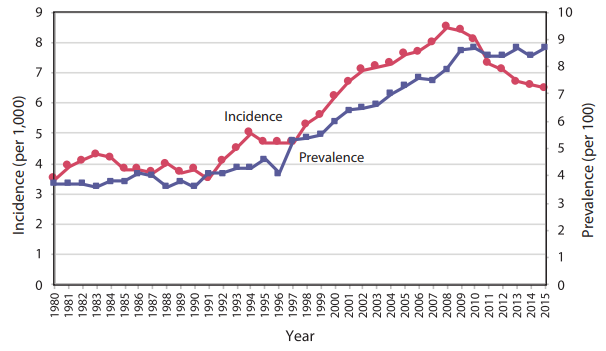
Figure 1 Trends in Incidence and Prevalence of Diagnosed Diabetes Among Adults Aged 18 or Older, United States, 1980–2015 5. Note: Diagnosed diabetes was based on self-report. Undiagnosed diabetes was based on fasting plasma glucose and A1C levels among people self-reporting no diabetes.
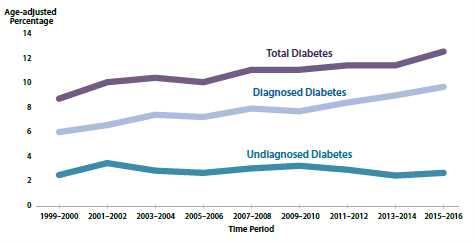
Figure 2. Trends in age-adjusted prevalence of diagnosed diabetes, undiagnosed diabetes, and total diabetes among adults aged 18 years or older, United States, 1999–2016 (2).
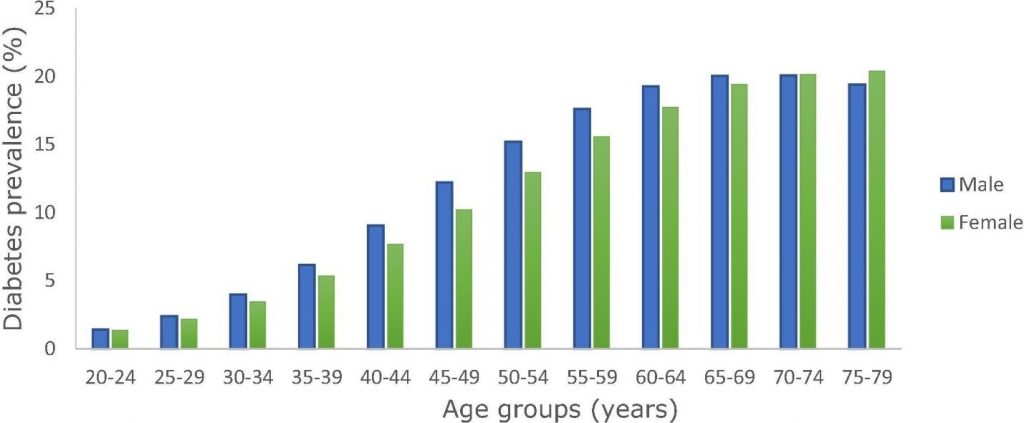
The worldwide prevalence of diabetes in women in 2019 is estimated to be 9.0%, and 9.6% in men (Figure 3).
Figure 3 Worldwide diabetes prevalence by age and sex in 2019 (4).
This growing prevalence of diabetes can be attributed to the longer life expectancy of the general population, improved detection techniques and diagnostic criteria (6), as well as a rise in both obesity and metabolic syndrome (7,8). As of 2016, more than 4,100 diabetes self-management education and support (DSMES) programs (with an estimated 1.1 million participants) were offered nationwide (2,5).
Childhood Type II DM has also increased over the past three decades, and seems to parallel the rising rates of childhood obesity (9,10). Of the estimated 34.2 million people diagnosed with diabetes, 210,000 are children and adolescents under 20 years, accounting for approximately 0.25 % of that population (2,5). Data from the SEARCH for Diabetes in Youth Study indicated that during 2014–2015, the estimated annual incidence of diagnosed diabetes in youth was estimated approximately 18,200 with type 1 diabetes, 5800 with type 2 diabetes (2). According to the National Health and Nutrition Examination Surveys (NHANES) the prevalence of obesity among U.S> youth ages 2-19 years was 18.5 % in 2015-2016. From 1999–2000 through 2015–2016, a significantly increasing trend in obesity was observed in both adults and youth. The observed change in prevalence between 2013–2014 and 2015–2016, however, was not significant among either adults or youth. Overall, the prevalence of obesity among adolescents (12–19 years) (20.6%) and school-aged children (6–11 years) (18.4%) was higher than among preschool-aged children (2–5 years) (13.9%) (11). Studies have shown a strong correlation between obesity and diabetes; specifically, 25% of obese children have an impaired glucose tolerance and 1% to 2% of obese children develop Type II DM. Thus, it’s not surprising that Type II DM accounts for nearly 45% of new cases of diabetes in the pediatric population, and that 2 million US adolescents under the age of 20 currently have impaired glucose tolerance (5).
In 2015, diabetes was the seventh leading cause of death in the US (5). The overall risk of death among diabetics is nearly twice that of people without diabetes (5). Diabetic complications affect both the macrovasculature (such as coronary artery disease and peripheral vascular disease) and the microvasculature (such as retinopathy, nephropathy, and neuropathy) (3); it also significantly impacts mortality rates. In 2016, a total of 7.8 million hospital discharges were reported with diabetes as any listed diagnosis among US adults aged 18 years or older (339.0 per 1,000 adults with diabetes). These discharges included 1.7 million in major cardiovascular diseases (75.3 per 1,000 adults with diabetes) and 168,000 for diabetic ketoacidosis (5). In addition, diabetes is responsible for 130,000 amputations (5), 11.7 % new cases of blindness (2), and over 500,000 hospitalizations each year.
These complications make diabetes an expensive disease, with an estimated annual cost of 327 billion dollars in 2017 (including 237 billion direct and 90 billion indirect medical costs). It is further estimated that 1 out of every 10 health care dollars is spent due to diabetes or its complications. In 2017, diabetes was the seventh leading cause of death in the United States. This finding is based on 83,564 death certificates in which diabetes was listed as the underlying cause of death (crude rate, 25.7 per 100,000 persons) (2).
Screening and Diagnosis:
The 2018 American Diabetes Association (ADA) recommended that testing for diabetes in all adults who are overweight or obese (BMI ≥25 kg/m2 or ≥23 kg/m2) and have one or more additional risk factors such as (12):
- Physical inactivity
- Family history of diabetes
- Member of a high-risk ethnic population (i.e., African American, Latino, Native American, Asian, or Pacific Islander)
- Hypertension (≥140/90 mmHg)
- HDL <35 mg/dl or triglycerides >250 mg/dl
- History of cardiovascular disease
- Age >45 years old
- Women with polycystic ovary syndrome
If the requirements for testing are met, a diagnosis of diabetes can be made in individuals who exhibit one of the following (13,14):
- Hemoglobin A1c ≥ 6.5%,
- Fasting plasma glucose ≥ 126 mg/dl
- 2-hour plasma glucose ≥ 200 mg/dl during an oral glucose tolerance test (OGTT)
- Classic symptoms of hyperglycemia plus a random plasma glucose ≥ 200 mg/dl.
In most instances, the diagnosis should be confirmed by repeat testing to rule out potential laboratory error, unless the diagnosis is clear on clinical grounds (13,14):
In some cases, a diagnosis of “prediabetes” can be made for patients whose glucose levels are too high to be considered normal but do not meet the criteria for diabetes. This does not indicate that a patient has the diabetes but implies that they are at an increased risk for diabetes and cardiovascular disease. A diagnosis of prediabetes can be made in individuals who exhibit:
- Hemoglobin A1c between 5.7-6.4%
- Fasting plasma glucose between 100 – 125 mg/dl
- 2-hour plasma glucose 140 -199 mg/dl
More information regarding the diagnosis and treatment of prediabetes and diabetes can be found on the American Diabetes Association website (professional.diabetes.org).
Diabetic Kidney Disease (Diabetic Nephropathy)
Prevalence and Screening:
Diabetic kidney disease (DKD), also known as diabetic nephropathy (DN), is defined as a microvascular complication of the kidneys induced by diabetes mellitus and is characterized by albuminuria in the absence of other renal disease and progressive loss of kidney function (15). Diabetes is the leading cause of CKD worldwide; in the US, it accounts for approximately 35- 50% of cases (16). DKD will develop in approximately 30% of type 1 diabetic patients and 40% of type 2 diabetic patients, and it is the single leading cause of end-stage renal disease (Figure 4) (17).
Figure 4 Trends in adjusted* ESRD incidence rate (per million/year), by primary cause of ESRD, in the U.S. population, 1996-2014. *Adjusted for age, sex, and race. The standard population was the U.S. population in 2011. Abbreviation: ESRD, end-stage renal disease.
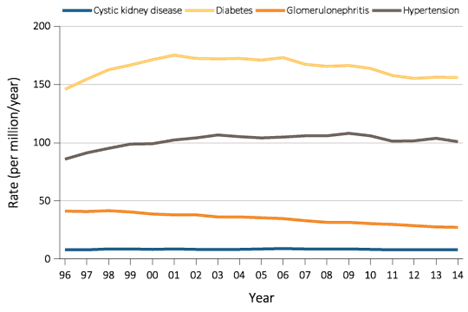
However, the updated report from USRDS (18) suggest that it is likely that the data from Centers for Medicare and Medicaid Services (CMS) 2728, which indicates diabetes as the primary cause of ESRD, may reflect the ESRD patients who have DM but not necessarily DM as the primary cause of their ESRD. This parallels the reports of biopsy-confirmed diabetic nephropathy, although there is likely selection bias in patients who undergo biopsy (19). Also, there may be a need to reclassify etiologies of ESRD that are listed on the form CMS 2728 to improve accuracy and to keep pace with scientific developments (20).
Screening for DKD is done through measurement of urinary albumin, as studies have shown proteinuria to be a strong predictor of renal injury and patient survival (21–23).
The American Diabetes Association (ADA) guidelines recommend assessment of urinary albumin (e.g., spot urinary albumin-to-creatinine ratio) and estimated glomerular filtration rate at least once a year in patients with type 1 diabetes with duration of ≥5 years, in all patients with type 2 diabetes, and in all patients with comorbid hypertension (24). Free screenings for high risk individuals can be obtained through the NKF’s Kidney Early Evaluation Program (KEEP). For more information on KEEP, please visit www.kidney.org.
The three most common methods for assessing urinary albumin are: (1) a 24-hour urine collection; (2) a timed urine collection (e.g., 4-hour or 10-hour overnight); or (3) measurement of the urine albumin-to-creatinine ratio in a random spot collection. The latter method is preferred because it is the most convenient and cost-effective (1). After the urine specimen is collected and evaluated, results may be classified as microalbuminuria (an albumin-to-creatinine ratio between 30 and 299 ug/mg) or microalbuminuria (an albumin-to-creatinine ratio ≥ 300 ug/mg). Classification should be confirmed with two additional samples collected during the next 3 to 6 month (24,25) period to rule out transient albuminuria (26).
The Joint Committee on Diabetic Nephropathy revised the classification of Diabetic Nephropathy (Classification of Diabetic Nephropathy 2014) in line with the widespread use of key concepts, such as the estimated glomerular filtration rate (eGFR) and chronic kidney disease (CKD). In revising the Classification, the Committee carefully evaluated, as relevant to current revision, the report of a study conducted by the Research Group of Diabetic Nephropathy, Ministry of Health, Labor and Welfare of Japan. Major revisions to the Classification are summarized as follows: (i) eGFR was substituted for GFR in the Classification; (ii) the subdivisions A and B in stage 3 (overt nephropathy) were reintegrated (Table 1); (iii) stage 4 (kidney failure) was redefined as a GFR <30 mL/min/1.73 m2, regardless of the extent of albuminuria; and (iv) stress was placed on the differential diagnosis of diabetic nephropathy versus non-diabetic kidney disease as being crucial in all stages of diabetic nephropathy.
Figure 5 Level of urinary albumin by various test methods and stage of CKD in diabetes. ACR, albumin to creatinine ratio; CKD, chronic kidney disease (27).
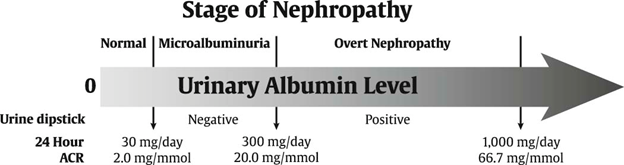
Table 1 Classification of Diabetic Nephropathy (28)
| Stage | Urinary albumin (mg/g Cr) or urinary protein (g/g Cr) | GFR (eGFR) (mL/min/1.73 m2) |
| Stage 1 (prenephropathy) | Normoalbuminuria (<30) | ≥30‡ |
| Stage 2 (incipient nephropathy) | Microalbuminuria (30–299)§ | ≥30 |
| Stage 3 (overt nephropathy)
(Subdivision A and B integrated) |
Macroalbuminuria (≥300) or
persistent proteinuria (≥0.5) |
≥30¶ |
| Stage 4 (kidney failure) | Any albuminuria/proteinuria status†† | <30 |
| Stage 5 (dialysis therapy) | Any status on continued dialysis therapy |
- †Diabetic nephropathy does not always progress from one stage to the next.
- ‡Although a glomerular filtration rate (GFR) of less than 60 mL/min/1.73 m2 is consistent with the diagnosis of chronic kidney disease, underlying causes other than diabetic nephropathy might be involved in patients with a GFR below 60 mL/min/1.73 m2, thus calling for the differential diagnosis between diabetic nephropathy and any other potential non-diabetic kidney diseases.
- §Patients with microalbuminuria are to be diagnosed as incipient nephropathy after the differential diagnosis based on the criteria for an early diagnosis of diabetic nephropathy.
- ¶Precautions are required in patients with macroalbuminuria, in whom renal events (e.g., a decrease in estimated GFR [eGFR] to half its baseline value, the need for dialysis) have been shown to increase as the GFR decreases below 60 mL/min/1.73 m2.
- ††All patients with a GFR of less than 30 mL/min/1.73 m2 are classified as showing kidney failure, regardless of their urinary albumin/protein values. However, in those with normoalbuminuria and microalbuminuria, the differential diagnosis is required between diabetic nephropathy and any other potential non-diabetic renal diseases.
Although macroalbuminuria is more closely associated with frank nephropathy, microalbuminuria is also an important marker of kidney damage and a predictor of cardiovascular events (25). Many individuals with Type II DM and microalbuminuria succumb to cardiovascular events before they progress to macroalbuminuria or renal failure (29). An association between microalbuminuria and cardiovascular risks such as lipid abnormalities, impaired endothelial function, peripheral vascular disease, and a pro-thrombotic state has been noted (26). Thus, in addition to renal dysfunction, microalbuminuria is a marker of generalized vascular dysfunction and endothelial injury (26).
Pathophysiology of Diabetic Kidney disease
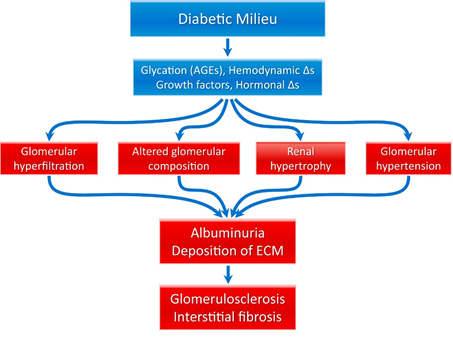
The pathophysiology of diabetic kidney disease is complex and multifactorial. Two mechanisms have been proposed, both of which are initiated by hyperglycemia (see figure 3). The first mechanism is based on hyperglycemia leading to protein glycosylation that in turn causes glomerular hypertrophy and eventually leads to sclerosis. The second proposes hyperglycemia leading to vasodilatation followed by hyperfiltration, abnormal angiotensin II response, abnormal endothelin/nitrous oxide (NO) response, increased growth hormone secretion, hyperinsulinemia and sclerosis (30–32). These lead to the release of reactive oxygen species and inflammatory mediators. Collectively, these changes result in glomerular hyperfiltration, glomerular hypertension, renal hypertrophy, and altered glomerular composition, which is manifested clinically as albuminuria and hypertension. Pathologically, the kidneys undergo several changes, including deposition (in primarily the mesangium) of extracellular matrix, glomerular basement membrane thickening, proliferative changes, and tubular atrophy, ultimately resulting in interstitial fibrosis and glomerulosclerosis (the final common pathway of many kidney diseases). A schema depicting this process is shown in Figure 6.
Figure 6 Pathophysiology of diabetic kidney disease (33).
Abbreviations: AGE, advanced glycation end product; ECM, extracellular matrix; ∆s, changes.
Pathological Classification:
In 2010, Tervaert et al., commissioned by the Research Committee of the Renal Pathology Society, proposed a pathologic classification system for DKD based on the severity of glomerular lesions (33). Under this classification system, glomerular biopsies are divided into one of four classifications, with Class I being mild nephropathy and Class IV being severe.
- Class I: Glomerular basement membrane (GBM) thickening: Isolated GBM (>395nm in women and > 430nm in men) thickening and only mild, nonspecific changes by light microscopy that do not meet the criteria of classes II through IV.
- Class IIa: Mild mesangial expansion, : Glomeruli classified as mild mesangial expansion (>25% of the observed mesangium that occupies an area smaller than the area of the capillary lumen) but without nodular sclerosis (Kimmelstiel–Wilson lesions) or global glomerulosclerosis in more than 50% of glomeruli.
- Class IIb: Severe mesangial expansion: Glomeruli classified as severe mesangial expansion (> 25% of the observed mesangium that occupies an area greater than the area of capillary lumen) but without nodular sclerosis and biopsy that does not meet criteria for class III or IV.
- Class III: Nodular sclerosis (Kimmelstiel–Wilson lesions): At least one glomerulus with nodular increase in mesangial matrix (Kimmelstiel–Wilson) without changes described in class IV.
- Class IV: Advanced diabetic glomerulosclerosis: More than 50% global glomerulosclerosis with other clinical or pathologic evidence that sclerosis is attributable to diabetic nephropathy and lesions from class I-III.
The proposed classification system may provide new insight in the complex pathways of DN. However, it should be noted that the study did not assess clinical outcomes because the researchers felt validation should be done in separate prospective studies with clearly defined clinical end points (25,33).
Treatment:
Optimal therapy of DKD continues to evolve. Guidelines from the ADA and NKF-KDOQI highlight the benefits of tight glycemic and blood pressure control as well as inhibition of the renin-angiotensin system in reducing the progression of diabetic kidney disease. Recently, it has been shown that intensive glucose control has not been shown to reduce the risk of DKD progression or improve clinical outcomes but instead increased the risk of hypoglycemia (28). The ADA recommends that glucose control targets should be individualized based on age, comorbidities, and life expectancy (34). At this time, KDOQI and KDIGO clinical practice guidelines recommend that the HbA1c target should be raised to >7% in patients with comorbidities, limited life expectancy, and those at risk for hypoglycemia, the latter of which include patients with advanced CKD, including those receiving dialysis (25). For hypertension management, Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: suggest that pharmacologic treatment to be initiated when systolic BP ≥ 130 mmHg or diastolic BP ≥ 80 mmHg (35). These BP targets are the same for CKD patients regardless of diabetes status (25). For patients with DKD, pharmacologic therapy should include an ACE inhibitor or an ARB alone or in combination with a medication from another BP class (25). Despite current therapy approaches to treatment of DKD, a focus of novel therapy options include targeting mechanisms such as glomerular hyperfiltration, inflammation and fibrosis, which could change the management of this disease state in the future (36). Managing other risk factors for cardiovascular disease, such as dyslipidemia, is also critical due to the high rate of cardiovascular death in this population (25,34). More information regarding the diagnosis and treatment of diabetic nephropathy can be found on the American Diabetes Association website (professional.diabetes.org) or the National Kidney Foundation (www.kidney.org).
The proliferation of HD regimens during the past few decades has generated much confusion and alphabet soup of terms without a firm structure or consistency.
References
- Trujillo J, Haines S. Diabetes Mellitus. In: DiPiro JT, Yee GC, Posey LM, Haines ST, Nolin TD, Ellingrod V, eds. Pharmacotherapy: A Pathophysiologic Approach, 11e. McGraw-Hill Education; 2020. Available from: http://accesspharmacy.mhmedical.com/content.aspx?aid=1166578968.
- Centre for Disease Control and Prevention. National Diabetes Statistics Report, 2020.Natl Diabetes Stat Rep. Published online 2020:2. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- Funk JL. Disorders of the Endocrine Pancreas. In: Hammer GD, McPhee SJ, eds.Pathophysiology of Disease: An Introduction to Clinical Medicine, 8e. McGraw-Hill Education; 2019. Available from: http://accessmedicine.mhmedical.com/content.aspx?aid=1156659591.
- Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9thedition. Diabetes Res Clin Pract. 2019;157. Available from: https://doi.org/10.1016/j.diabres.2019.107843.
- Centers for Disease Control and Prevention. Diabetes Report Card, 2017. Atlanta,GA. Available from: https://www.cdc.gov/diabetes/pdfs/library/diabetesreportcard2017-508.pdf.
- Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988-2012.JAMA. 2015;314(10):1021-1029. Available from: https://pubmed.ncbi.nlm.nih.gov/26348752.
- Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997-2003.Am J Prev Med. 2006;30(5):371-377. Available from: https://pubmed.ncbi.nlm.nih.gov/16627124.
- Moore PA, Zgibor JC, Dasanayake AP. Diabetes: a growing epidemic of all ages.J Am Dent Assoc. 2003;134 Spec N:11S-15S. Available from: https://pubmed.ncbi.nlm.nih.gov/18196668.
- Reinehr T. Type 2 diabetes mellitus in children and adolescents.World J Diabetes. 2013;4(6):270-281. Available from: https://pubmed.ncbi.nlm.nih.gov/24379917.
- Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M. Type 2 Diabetes in the Young: The Evolving Epidemic.Diabetes Care. 2004;27(7):1798 LP – 1811. Available from: http://care.diabetesjournals.org/content/27/7/1798.abstract.
- NCHS Fact Sheet, December 2017. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/nchs/about/factsheets/factsheet_nhanes.htm.
- American Diabetes Association. Standards of medical care in diabetes–2006.Diabetes Care. 2006;29 Suppl 1:S4-42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16373931.
- Pippitt K, Li M, Gurgle HE. Diabetes Mellitus: Screening and Diagnosis.Am Fam Physician. 2016;93(2):103-109. Available from: https://pubmed.ncbi.nlm.nih.gov/26926406.
- Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: Recommendation Statement.Am Fam Physician. 2016;93(2). Available from: https://pubmed.ncbi.nlm.nih.gov/26926415.
- Reutens AT. Epidemiology of Diabetic Kidney Disease.Med Clin North Am. 2013;97(1):1-18. Available from: http://www.sciencedirect.com/science/article/pii/S0025712512001861.
- Usrds.Chapter 1: Incidence, Prevalence, Patient Characteristics, and Treatment Modalities. Available from: https://www.usrds.org/2018/download/v2_c01_IncPrev_18_usrds.pdf.
- United States Renal Data Systems.2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. Available from: www.usrds.org.
- United States Renal Data System. 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2017.
- Layton JB, Hogan SL, Jennette CE, et al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases.Clin J Am Soc Nephrol. 2010;5(11):2046-2052. Available from: https://pubmed.ncbi.nlm.nih.gov/20688886.
- Tucker BM, Freedman BI. Need to Reclassify Etiologies of ESRD on the CMS 2728 Medical Evidence Report.Clin J Am Soc Nephrol. 2018;13(3):477 LP – 479. Available from: http://cjasn.asnjournals.org/content/13/3/477.abstract.
- Borch-Johnsen K, Andersen PK, Deckert T. The effect of proteinuria on relative mortality in type 1 (insulin-dependent) diabetes mellitus.Diabetologia. 1985;28(8):590-596. Available from: https://pubmed.ncbi.nlm.nih.gov/4054448.
- Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in Type 1 (insulin-dependent) diabetes: an epidemiological study.Diabetologia. 1983;25(6):496-501. Available from: https://pubmed.ncbi.nlm.nih.gov/6363177.
- de Zeeuw D, Remuzzi G, Parving H-H, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL.Kidney Int. 2004;65(6):2309-2320. Available from: https://pubmed.ncbi.nlm.nih.gov/15149345.
- Microvascular Complications and Foot Care: <em>Standards of Medical Care in Diabetes—2019</em>Diabetes Care. 2019;42(Supplement 1):S124 LP-S138. Available from: http://care.diabetesjournals.org/content/42/Supplement_1/S124.abstract.
- National Kidney Foundation. K/DOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update.Am J Kidney Dis. 2012;60(5):850-886. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0272638612009572.
- Mogensen CE, Vestbo E, Poulsen PL, et al. Microalbuminuria and potential confounders. A review and some observations on variability of urinary albumin excretion.Diabetes Care. 1995;18(4):572-581. Available from: https://pubmed.ncbi.nlm.nih.gov/7497874.
- Mcfarlane P, Cherney D, Gilbert Mbbs RE. Chronic Kidney Disease in Diabetes. Published online 2018. Available from: https://doi.org/10.1016/j.jcjd.2017.11.004.
- Haneda M, Utsunomiya K, Koya D, et al. A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy.J Diabetes Investig. 2015;6(2):242-246. Available from: https://pubmed.ncbi.nlm.nih.gov/25802733.
- Lewis JB, Neilson EG. Glomerular Diseases. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds.Harrison’s Principles of Internal Medicine, 20e. McGraw-Hill Education; 2018. Available from: http://accessmedicine.mhmedical.com/content.aspx?aid=1156520317.
- Epstein M, Sowers JR. Diabetes mellitus and hypertension.Hypertens (Dallas, Tex 1979). 1992;19(5):403-418.
- Sowers JR, Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update.Hypertens (Dallas, Tex 1979). 1995;26(6 Pt 1):869-879.
- Nathan DM. Long-term complications of diabetes mellitus.N Engl J Med. 1993;328(23):1676-1685.
- Umanath K, Lewis JB. Update on Diabetic Nephropathy: Core Curriculum 2018.Am J Kidney Dis. 2018;71(6):884-895. Available from: https://doi.org/10.1053/j.ajkd.2017.10.026.
- Introduction: <em>Standards of Medical Care in Diabetes—2020</em>Diabetes Care. 2020;43(Supplement 1):S1 LP-S2. Available from: http://care.diabetesjournals.org/content/43/Supplement_1/S1.abstract.
- Cifu AS, Davis AM. Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.JAMA. 2017;318(21):2132-2134. Available from: https://doi.org/10.1001/jama.2017.18706.
- Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: Challenges, progress, and possibilities.Clin J Am Soc Nephrol. 2017;12(12):2032-2045.
P/N 101290-01 Rev A 3/2020
