Clinical Outcomes of PD and HD
Mortality Trends in Peritoneal Dialysis and Hemodialysis
The total life expectancy of an individual with end stage renal disease (ESRD) is only one-fourth to one-fifth that of the general population (1). This difference in survival can be attributed to the higher rates of cardiovascular and infectious complications associated with ESRD (1). Despite these risks, the overall mortality rates of peritoneal dialysis (PD) and hemodialysis (HD) have considerably improved during the past two decades. Advancing technologies, earlier referral, and better management of comorbid disease states and infections have all positively impacted the mortality rate of the dialysis population. The rate of PD mortality, in particular, has fallen more rapidly compared to HD due to major improvements in PD delivery, efficacy, and safety (1, 2) [see Figure 1]. Furthermore, adjusted five-year survival on PD has improved 7.5% between the 1992-1996 and 1997-2001 periods, and is now similar for HD and PD patients at 33.5% and 33.9%, respectively (1) [see Figure 2].
Although the adjusted survival of PD and HD over the total 5-year period is almost identical, the relative risk of death between the two modalities appears to change over time on dialysis. Several recent studies indicate that PD is associated with better survival during the first 1-2 years of dialysis whereas HD is associated with better survival thereafter (3-12). Several explanations for this shift have been proposed including: a reduced rate of loss of residual renal function in patients on PD and a greater level of comorbidity among HD patients at initiation (3) seems to benefit early PD survival, where as technique failure due to recurrent peritonitis and loss of ultrafiltration with the increase in peritoneal membrane transport characteristics (8) and less frequent monitoring of PD patients by their nephrologistsmight be factors becoming adversely relevant after the first few years on PD. Apart from general difference between treatment modalities, survival is also dependent on other patient specific influential factors such as age, gender, race, body weight, and educational status. Understanding these subgroup differences and mortality trends is essential for optimizing patient outcomes.
Due to the difference in early and late survival, some have suggested using a “dual-modality” or “integrative-care” approach with initiation of PD, followed by timely transfer to HD. A study by Van Biesen et al. showed a survival advantage in a matched-pair analysis of patients who started on PD and were transferred to HD, versus patients who started and remained on HD (4). Similarly, a retrospective analysis of data from China on incident patients treated with either PD or HD also found that initial treatment with PD was associated with a higher survival rate (13). Yet, a study by Traynor et al. reported that initial dialysis modality was not a significant predictor of survival after adjusting for age, sex, and primary renal diagnosis (14). Thus, in the absence of randomized controlled studies, definite recommendations regarding the dialysis modality based on mortality rates cannot be made, even though some data seems to suggest that starting patients on PD might be beneficial. The following discussion on factors relevant for patient outcome may provide some insight into the growing body of research that continues to dissect PD and HD mortality rates.
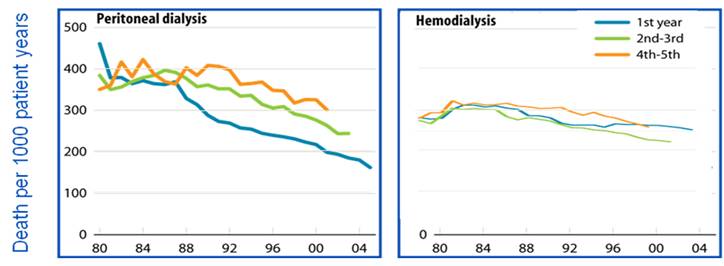
Figure 1: USRDS 2008 (incident ESRD patients; adjusted for age, gender, race and primary diagnosis. Incident ESRD patients, 2005, used as reference cohort)
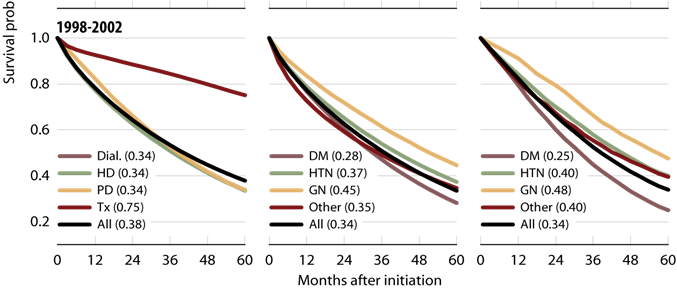
Figure 2: USRDS 2009 5 Year Survival Probability (adjusted for age, gender, & race; primary diagnosis. All ESRD patients, 2005, used as reference cohort)
Influential Characteristics Affecting PD & HD Mortality
Age:
Recent data indicate that age, in the presence of comorbid conditions, plays a significant role in dialysis mortality (3, 7, 9-12). A study by McDonald et al. suggested that PD was more beneficial in younger patients (< 60 y/o) without comorbidities, while HD was more beneficial in older patients (11). Weinhandle et al. also found a survival benefit in PD patients under 65 y/o without comorbid conditions (12). Vonesh et al. showed that PD was associated with better outcomes in younger diabetic patients (< 45 y/o) with fewer comorbidities, while HD was superior among older diabetic patients (> 45 y/o) regardless of comorbidities (7). Taken together, these studies suggest that PD is associated with better outcomes among younger populations having fewer comorbid conditions, whereas being older and having multiple comorbid conditions favors HD.
Consideration for age differences is important because individuals receiving PD tend to be younger than those receiving HD. Therefore, adjustment for age is essential when comparing mortality data. Specifically, 78% of PD patients are less than 65 years old compared to 55% of HD patients (15). In addition, new ESRD patients who choose PD as their initial treatment modality also tend to be younger than those starting HD (15). Therefore, the current age distribution of the dialysis population suggests that PD patients are at an inherently lower risk of death compared those using HD. The subsequent affect of age and modality selection on survival also furthers the need for an “integrative-care” approach.
The above data has not been proven in all studies, although other studies have shown similar results (3, 6, 10). A meta-analysis performed in 2001 by Selgas et al. suggests that the mortality and hospitalization rates in elderly dialysis patients (>65 y/o) are not significantly different between PD and HD (16). However, several notable aspects of both modalities have not been compared in the elderly such as progression of atherosclerosis, the influence of plasma lipid profile, homocysteine levels, and frequency of sudden death/arrhythmia (16).
Gender:
Unlike the general population, in which females have a higher life expectancy than males, recent data suggests that females with ESRD experience lower rates of survival (1). The most compelling difference in expected remaining life-times between the general and ESRD populations is found among female dialysis patients in their 30s, 40s, and 50s, who are expected to live just one-fifth as long as their counterparts without ESRD (1). Villar et al. noted that standard mortality ratios were 1.5 times higher in women than men in the first four years after dialysis onset. The authors concluded that dialysis therapy “cancels out” the survival advantage that women in the general population experience (17). Additionally, other studies suggest that diabetic women experienced greater mortality compared to diabetic men, especially when using PD (3, 18, 19). The reasons for this mortality difference are not completely understood, however female patients may be more likely to develop cardiovascular disease, obesity, protein-energy wasting (19), and infections (20) compared to their male counterparts. Additionally, female diabetic patients in these studies tended to have a lower residual renal function at the start of PD therapy (19).
Considering that 48% of prevalent PD patients are female versus 45% of HD patients (15), together with the fact that female patients are more likely to choose PD as their initial dialysis modality (15), continued research will certainly benefit this vulnerable population.
Ethnicity & Race:
To date, no study has directly compared the mortality rates of ethic groups undergoing PD therapy versus those undergoing HD therapy. The statistical challenge of such a study may be compounded by minimal data sets and/or misclassification of minority groups in administrative databases (21). Though patient ethnicity has been a required data field on the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Form (MEF; Form 2728) since 1995, only the ESRD Clinical Performance Measures (CPM) form specifically lists the subgroups of non-Hispanic, Hispanic–Mexican, American (Chicano), Hispanic–Puerto Rican, Hispanic–Cuban American, and Hispanic other. In the 2007 ESRD CPM project report, several tables describe important clinical characteristics of adult in-center hemodialysis and peritoneal dialysis patients for various race groups; however, the comparisons are limited to White vs. Black due to inadequate sample size (21).
Nevertheless, multiple studies have been published comparing mortality between minority races and Caucasians. These studies demonstrate that ethnic minorities have a survival advantage compared to Caucasian dialysis patients. Astudy by Frankenfield et al. and a separate study by Murthy et al. both show that Hispanics have a survival advantage over non-Hispanics, although it varied by racial/ethnic subgroup (21,22). The authors noted that a greater dose of delivered dialysis, younger age, lower comorbidity burden, and higher serum albumin and hemoglobin values in the Hispanic population may partially explain the survival advantage in this study. Another cohort study by Eisenstein et al. showed that African-American HD patients of all socioeconomic status exhibited higher survival compared to other races (23). Similarly, in a study of 3700 PD and HD patients, Pei and colleges reported that Caucasians had a higher incidence of death compared to Asians and Black patients (24). Such differences may be due to a higher proportion of white smokers with symptomatic cardiovascular disease and a higher average dialysis dose in the Asian population due to their smaller body size (24). The genetic differences between races have also been a proposed reason for the improved survival of ethnic minorities (25).
However, these differences have not been confirmed in all studies. Using a more comprehensive covariant adjustment as compared to previous studies, Robinson et al. reported marked attenuation of the survival advantage in Hispanics, Blacks, Asians, and Native Americans. Therefore, he concluded that minorities should not be expected to survive longer on HD than non-Hispanic white patients with similar clinical attributes. He also cautioned that the identification of variables that explain the statistical associations of race/ethnic category with survival may be a biased method to understand the reasons for differences in survival by race and ethnicity (26).
The data suggest that ethnic minorities undergoing dialysis might have a different survival than Caucasian dialysis patients. Since more HD patients tend to be of ethnic origin (African American, Native American, Asian, and Hispanic) compared to PD patients (15), ethnicity must be adjusted for when comparing the survival between these groups.
Body Mass Index (BMI)
Obesity is often cited as a risk factor for vascular disease and a relative contraindication for PD. Interestingly, increased body weight and body mass index (BMI) have been identified as potentially protective factors, improving survival in HD and to a lesser extent in PD patients. This “reverse epidemiology” seen in HD has been studied prospectively by Kalantar-Zadeh et al. who examined the changes in weight over time and prospective mortality using time-dependent Cox models with adjustments for changes in laboratory values and BMI with mortality (27). Weight gain and both baseline and time-varying obesity were associated with reduced cardiovascular mortality in HD patients, independent of laboratory findings and nutritional status. In fact, morbidly obese patients had the lowest mortality (27).Similar results were noted in several other studies of obese HD patients (28-30). Obese patients may experience better survival for a number of reasons. One explanation is that obese patients tend to have higher short-term nutritional markers, such as pre-albumin and transferrin. Obese patients also have a higher calorie intake, which may suppress protein catabolism and reduce the endogenous uremic load. In addition, larger patients may be better able to dilute uremic toxins compared to those with a normal BMI (30).
The positive benefits of obesity on survival have not been consistently proven in PD patients. While some studies have associated obesity with a survival advantage in PD (31-33), others have shown a neutral benefit to mortality (28, 34, 35) or greater mortality (36). The reasons for these conflicting results are complex but are likely due to differences in study design. Furthermore, differences in outcomes between obese PD and obese HD patients may be explained by: (1) a possible reduction in overall dialysis dose in obese PD patients compared to obese HD patients (28); (2) a more rapid loss of residual renal function in obese PD patients compared to normal PD patients and obese HD patients (36); and (3) the negative effect of dextrose-based PD solutions on lipid and glycemic profiles (34). The study of PD and obesity is also confounded by the fact that nephrologists are less likely to recommend PD as an option for patients with a larger body mass, due to the concern for achieving adequate dialysis (37, 38).
Thus, data suggests that obesity confers a survival advantage in HD, whereas the data on PD is still conflicting. However, the use of BMI as a measure of obesity is not without its limitations. BMI may overestimate adiposity on those with more lean body mass (ex-athletes) and makes simplistic assumptions about the distribution of muscle and bone. Some studies have actually found that gaining muscle mass rather than fat mass has improved PD outcomes (39). Furthermore, while desired weight may vary with ethnicity, sex, and age, the typical cutoffs used to define normal, overweight, and obese make no allowances for these variations (30). The distribution of body fat must also be taken into account since truncal obesity has the potential to negatively impact transplant status and is largely associated with inflammation, protein energy wasting, and increased mortality (40).
Educational Background & Health Literacy
The educational background of PD and HD patients is an important predictor of survival and treatment success. Those with a better education are likely to possess better health literacy, thereby giving them the cognitive and social skills needed to gain access to, understand, and use health information (41, 42). Numerous studies have shown that low health literacy directly correlates with poorer health status, lack of knowledge about medical care, decreased comprehension of medical information, poorer self-reported health, poorer compliance rates, increased hospitalizations, and increased health care costs (43). Similar reports suggest that people who read at lower levels are generally 1.5 to 3 times more likely to have an adverse outcome compared to those who read at higher levels (44).
Consideration for health literacy is especially important in the dialysis population due to educational disparities between PD and HD patients. Individuals who completed high school or graduated from college have a greater likelihood of placement on PD (37, 45). Thus, PD patients may need to be more competent and knowledgeable in order to understand the complexities of their procedure (37). PD patients may also be more assertive and autonomous in the decision making process compared to those receiving HD (37). Therefore, educational status is an important confounding variable that should be taken into consideration when comparing dialysis outcomes.
Comorbid conditions
Several studies have concluded that the differences in survival observed between HD and PD patients are highly dependent on the frequency and severity of comorbid factors.
Malnutrition
Malnutrition is common in ESRD patients and is a powerful predictor of morbidity and mortality. PD patients may be severely hypoalbuminemic at the initiation of dialysis (46)and may potentially remain at higher risk of malnutrition due to constant protein losses in the effluent that are significantly higher among high peritoneal transporters and during episodes of peritonitis. In the ESRD population, nutritional status requires close monitoring and consideration of proper nutritional supplements. Although much progress has been made in recent years with respect to identifying the causes and pathogenesis of malnutrition in ESRD patients, clinical management of malnutrition remains a great challenge for the nephrologists and patients.
Diabetes
Diabetic nephropathy is the single leading cause of end-stage renal disease, accounting for 54% of new cases in the United States in 2007 (47). In addition, the survival of diabetic ESRD patients remains the lowest among all primary diagnoses, likely due to the high prevalence of cardiovascular disease in this population (1, 19). However, the probability of survival for a diabetic patient seems to change depending on the treatment modality chosen and other patient characteristics including age and underlying comorbidities.
A study by Lin et al. showed that non-diabetic PD patients had a survival advantage over non-diabetic HD patients. Furthermore, of the patients with diabetes, PD patients had better outcomes than HD (13). Vonesh et al. also showed that PD was associated with better outcomes in younger diabetic patients (< 45 y/o) with few comorbidities, while HD was superior among older diabetic patients (>45 y/o) regardless of comorbidities (7). Other studies further support the notion that PD confers better outcomes over HD in non-diabetics and younger diabetics with fewer comorbidities (3, 6, 10, 48).
The reasons for the differences in diabetic survival have not yet been proven. Studies have shown that during the first 3 months of dialysis, diabetic patients who start HD may switch to PD due to vascular access complications (49). However, switch of diabetics to PD due to problems such as poor venous access or hemodynamic instability may be a signal of emerging comorbidity, and might explain the higher mortality associated with PD especially in older diabetics (7). PD may confer a survival advantage to younger diabetics due to better preservation of residual renal function compared to those using HD (7). Similarly, compared to older diabetics, young diabetic PD patients can better metabolize and utilize daily glucose loads from glucose-containing PD solutions (7).
Such differences highlight the potential benefit of an integrative-care approach, which may contribute to optimized patient outcomes and survival (4, 13).
Cardiovascular disease
Cardiovascular disease (CVD) is the leading cause of mortality and hospitalization in ESRD patients (1) due to accelerated atherogenesis, lipid derangements, endothelial dysfunction, and inflammation (50). Pre-existing cardiovascular conditions may worsen outcomes in new ESRD patients, depending on the treatment modality chosen.
Ganesh et al. showed that patients with pre-existing cardiovascular conditions, such as coronary artery disease (CAD), peripheral vascular disease, arrhythmias, and heart failure, have a higher risk of mortality compared to those without these conditions at ESRD onset (50). The researchers noted that CAD patients treated with PD had a higher proportion of deaths compared to HD-treated patients after 6 months of therapy. Furthermore, among patients with cardiovascular disease in a national prospective cohort study by Jaar et al., the risk for death was approximately twice as high in those undergoing PD than in those undergoing hemodialysis. However, among patients without cardiovascular disease, no increased risk for death was observed among patients undergoing PD (8). Other studies have shown similar results (51).
There are good arguments supporting that, at least theoretically, PD should offer patients with cardiac conditions improved blood pressure control, less hemodynamic stress, and avoidance of the uremic peaks and troughs often seen with HD (50). To the contrary, the above-mentioned evidence suggest that PD possibly worsens survival of those with CVD through a number of mechanisms. One theory is that PD promotes a pro-atherogenic environment, since patients with PD tend to have higher levels of serum triglycerides, total cholesterol, and LDL, as well as lower levels of HDL compared to HD treated patients (50). It has also been suggested that ultrafiltration failure and the development of high transport membrane permeability over time may contribute to hypertension, fluid overload, and cardiomyopathy in PD-treated patients (50). Additionally, PD patients are seen less frequently by their nephrologists compared to HD patients, which may mean that less attention is given to cardiovascular risk factors. Other reasons include differences in homocysteine levels, oxidative stress, and soluble adhesion molecules between PD and HD patients that may further stimulate underlying atherogenesis (50).
Quality of life
In addition to the factors listed above, a lower health-related quality of life (HRQOL) has been shown to predict morbidity and mortality in ESRD patients (52, 53). Thus, assessing the HRQOL for each treatment modality is critical in achieving positive outcomes. Unfortunately, data comparing the QOL between PD and HD have been inconsistent. While some studies show a higher QOL for PD patients compared to HD, others have found no such difference (54). These results are best summarized by a recent meta-analysis of 27 studies comparing four different quality of life utility measures. Overall, the analysis showed that there was no statistically significant difference in quality of life between HD and PD patients (54).
However, it cannot be denied that home HD and PD therapies offer patients more freedom and independence compared to in-center dialysis. Furthermore, a growing body of evidence suggests that patients undergoing daily home HD have lower adjusted mortality rates compared to those who dialyze in-center (55). Using nocturnal hemodialysis 3-7 nights each week for 6-8 hours has been associated with an improvement in cardiovascular outcomes and calcium-phosphate metabolism, a decrease in erythropoietin dose, and a survival rate similar to that of patients receiving a deceased donor kidney transplant (56, 57). Such benefits are certainly due to the increased frequency and duration of dialysis treatment that inadvertently improves the removal of unwanted toxins (57). A better QOL with dialysis at home might be part of this beneficial effect; however, there is no quantitative evidence to what extent this might apply.
Quality of care
The quality of general and nephrological care will logically affect the outcomes of dialysis. Inadequate training of the clinician or patient will lead to high rates of technique failure and mortality (58). Schaubel et al. identified center experience in PD as having a significant impact on the relative risk of death and technique failure. Comparing centers with the most and the least cumulative experience of PD, patients treated at more experienced centers had a 30% reduction in risk of death. Risk of technique failure was also diminished by PD experience (17% reduction for most versus least experienced) (59). Clinician knowledge and training has historically focused more on HD than PD (60, 61, 62); however, this trend appears to be changing with the increased focus on dual and home therapies.
Quality of care involves early referral to a nephrologist before the onset of ESRD. Patients referred earlier are more likely to have better metabolic status, greater serum albumin and hemoglobin levels, earlier establishment of permanent vascular access, less cardiovascular disease, shorter initial hospitalizations, and received treatments such as erythropoietin, vitamin D supplements, phosphate binders, and antihypertensive therapy (63). Early referral provides the time for education before frank uremia ensues, allowing the patient to participate in the choice of therapy modality that best suits his or her individual lifestyle and promoting self therapy (64). In patients who chose HD, Lorenzo et al. noted that 73% received timely placement of an arterio-venous fistula (AVF) if seen by a nephrologist > 3 months prior to dialysis initiation (65). AVFs have the lowest complication rates, require fewer procedures, and have lower expenditures than arterio-venous grafts or central venous catheters.
Consideration of practitioner and center experience, as well as early referral to a nephrologist strongly impact PD and HD outcomes.
Summary
The overall mortality rate of peritoneal dialysis (PD) and hemodialysis (HD) has considerably improved during the past two decades. The rate of PD mortality, in particular, has fallen more rapidly compared to HD due to major improvements in PD delivery, efficacy, and safety resulting in a similar adjusted five-year survival in HD and PD patients at 33.5% and 33.9%, respectively. While both modalities have the same five-year survival, several recent studies seem to indicate that PD is associated with better survival during the first 1-2 years of dialysis, whereas HD is associated with better survival thereafter. In addition to these general findings, individual patient outcome is determined by patient characteristics that affects PD and HD mortality. Age, gender, ethnicity, race, body mass index, educational background, health literacy, and the patient’s comorbid conditions are patient-specific variables that influence outcome on HD or PD treatment. Choosing the right patient for the right modality has been shown to have a great impact not only on health-related outcomes but on quality of life. Thus, it is important to consider the social implications of each modality on the patient’s life. Independent from patient characteristics and preference, the quality of general and nephrological care will affect the outcomes of dialysis. Clinical knowledge and training have historically focused more on center HD than PD. Many nephrology training programs do not offer trainees sufficient exposure to home therapies. Accordingly, center experience has shown to significantly impact the relative risk of death and technique failure in PD. Thus, adequate training and education of patients and health care professionals, together with thoroughly considering the impact of patient characteristics and patient preference is the key to choosing dialysis modality.
Reference:
- United States Renal Data System 2009. Chapter 6: Morbidity and Mortality. Retrieved 10/07, 2020, from https://www.usrds.org/annual-data-report/current-adr/
- Krediet R. Advances in peritoneal Dialysis: Towards Improved Efficacy and Safety. Blood Purif 16:1-14, 1998
- Collins AJ, Hao W, Xia H, Ebben JP, Everson SE, Constantini EG, Ma JZ. Mortality Risks of Peritoneal Dialysis and Hemodialysis. Am J Kidney Dis 36:1065-1074, 1999
- Van Biesen W, Vanholder RC, Veys N, Dhondt A, Lameire NH. An Evaluation of an Integrative Care Approach for End Stage Renal Disease Patients. J Am Soc Nephrol 11:116-125, 2000
- Heaf J, Lokkegaard H, Madsen M. Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17: 112-117, 2002
- Termorshuizen F, Korevaar JC, Dekker FW, Van Manen JG, Boeschoten EW, Krediet RT; Netherlands Cooperative Study on the Adequacy of Dialysis Study Group. Hemodialysis and Peritoneal Dialysis: Comparison of Adjusted Mortality Rates According to the Duration of Dialysis: Analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis 2. J Am Soc Nephrol 14: 2851-2860, 2003
- Vonesh E, Snyder J, Foley R, Collins A. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney International 66: 2389-2401, 2004
- Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, Levin NW, Sadler JH, Kliger A, Powe NR. Comparing the Risk for Death with Peritoneal Dialysis and Hemodialysis in a National Cohort of Patients with Chronic Kidney Disease. Ann Intern Med 143:174-183, 2005
- Vonesh EF, Snyder JJ, Foley RN, Collins AJ. Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney International 70:S3-S11, 2006
- Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC. Comparison of hemodialysis and peritoneal dialysis survival in The Netherlands. Kidney International 71:153-158, 2007
- McDonald S, Marshall M, Johnson DW, and Polkinghorne K. Relationship between Dialysis Modality and Mortality. J Am Soc Nephrol 20:155-163, 2009
- Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ. Propensity-Matched Mortality Comparison of Incident Hemodialysis and Peritoneal Dialysis Patients. J Am Soc Nephrol 21:499-506, 2010
- Lin TC, Kao MT, Lai MN, Huang CC. Mortality Difference by Dialysis Modality Among New ESRD Patients with and without Diabetes Mellitus. Dialysis & Transplantation 35:234-244, 2006
- Traynor J, Simpson K, Geddes C. Early Initiation of Dialysis Fails to Prolong Survival in Patients with End-Stage Renal Failure. J Am Soc Nephrol 13:2125-2132, 2002
- United States Renal Data System 2009. Chapter 4: Treatment Modalities. Retrieved 10/07, 2020, from https://www.usrds.org/annual-data-report/current-adr/
- Selgas R, Cirugeda A, Fernandez-Perpén A, Sánchez-Tomero JA, Barril G, Alvarez V, Bajo MA. Comparisons of hemodialysis and CAPD in patients over 65 years of age: A meta-analysis. Int Urol Nephrol 33:259-264, 2001
- Villar E, Remontet L, Labeeuw M, Ecochard R. Effect of Age, Gender, and Diabetes on Excess Death in End-Stage Renal Disease. J Am Soc Nephrol 18: 2125-2134, 2007
- Karamé A, Labeeuw M, Trolliet P, Caillette-Beaudoin A, Cahen R, Ecochard R, Galland R, Hallonet P, Pouteil-Noble C, Villar E. The Impact of Type 2 Diabetes on Mortality in End-Stage Renal Disease Patients Differs between Genders. Nephron Clin Pract 112:c268-c275, 2009
- Chung SH, Han DC, Noh H, Jeon JS, Kwon SH, Lindholm B, Lee HB. Risk factors for mortality in diabetic peritoneal dialysis patients. Nephrol Dial Transplant, 2010. Retrieved 7/23/2010 from https://www.ncbi.nlm.nih.gov/pubmed/20466690
- Piraino B, Bernardini J, Centa PK, Johnston JR, Sorkin MI. The Effect of Body Weight on CAPD Related Infections and Catheter Loss. Perit Dial Int 11: 64-68, 1991
- Frankenfield DL, Krishnan SM, Ashby VB, Shearon TH, Rocco MV, Saran R. Differences in Mortality Among Mexican-American, Peurto Rican, and Cuban-American Dialysis Patients in the United States. Am J Kidney Dis 53: 647-657, 2009
- Murthy B, Molony D, Stack A. Survival Advantage of Hispanic Patients Initiating Dialysis in the United States Is Modified by Race. J Am Soc Nephrol 16: 782-790, 2005
- Eisenstein EL, Sun JL, Anstrom KJ, Stafford JA, Szczech LA, Muhlbaier LH, Mark DB. Do Income Level and Race Influence Survival in Patients Receiving Hemodialysis? Am J Med 122: 170-180, 2009
- Pei Y, Greenwood C, Chery A, Wu G. Racial differences in survival of patients on dialysis. Kidney Int 58: 1293-9, 2000
- Ogata S, Yorioka N, Gilbertson DT, Chen SC, Foley RN, Collins AJ. Genetic and environmental effects and characteristics of Japanese end-stage renal disease patients. Hemodial Int 13 Suppl 1: S8-12, 2009
- Robinson B, Joffe M, Pisoni R, Port F, Feldman H. Hemodialysis Patients : The Dialysis Outcomes and Practice Patterns Study. J Am Soc Nephrol 17: 2910-2918, 2006
- Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, Greenland S. Association of Morbid Obesity and Weight Change Over Time with Cardiovascular Survival in Hemodialysis Population. Am J Kidney Dis 46: 489-500, 2005
- Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL. Body mass index, dialysis modality, and survival: Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65: 597-605, 2004
- Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 16: 2386-94, 2001
- Schmidt D, Salahudeen A. Obesity-Survival Paradox—Still a Controversy? Semin Dial 20:486-92, 2007
- Johnson DW, Herzig KA, Purdie DM, Chang W, Brown AM, Rigby RJ, Campbell SB, Nicol DL, Hawley CM. Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 20:715-21, 2000
- Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int 64: 1838-1844, 2003
- Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 80: 324-32, 2004
- Aslam N, Bernardini J, Fried L, Piraino B. Large body mass index does not predict short-term survival in peritoneal dialysis patients. Perit Dial Int 22:191-196, 2002
- de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contrib Nephrol 163:124-31, 2009
- McDonald S, Collins J, Johnson D. Outcomes in the Australia and New Zealand Patient Populations. J Am Soc Nephrol 14: 2897-2901, 2003
- Stack A. Determinants of Modality Selection among Incident US Dialysis Patients: Results from a National Study. J Am Soc Nephrol 13: 1279-1287, 2002
- Thamer M, Hwang W, Fink NE, Sadler JH, Wills S, Levin NW, Bass EB, Levey AS, Brookmeyer R, Powe NR. US Nephrologists’ Recommendation of Dialysis Modality: Results of a National Survey. Am J Kidney Dis 36:1155-65, 2000
- Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of Body Size and Body Composition on Survival in Hemodialysis Patients. J Am Soc Nephrol 14: 2366-2372, 2003
- Cordeiro AC, Qureshi AR, Stenvinkel P, Heimbürger O, Axelsson J, Bárány P, Lindholm B, Carrero JJ. Abdominal fat deposition is associated with increased inflammation, protein-energy wasting, and worse outcome in patients undergoing haemodialysis. Nephrol Dial Transplant 25: 562-568, 2010
- Nutbeam D. The evolving concept of health literacy. Soc Sci Med 67:2072-8, 2008
- Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promotion International 15:259-267, 2000
- Andrus MR, Roth MT. Health literacy: a review. Pharmacotherapy 22: 282-302, 2002
- Dewalt DA, Berkman ND, Sheridan S, Lohr KN, Pignone MP. Literacy and Health Outcomes. J Gen Intern Med 19: 1228-1239, 2004
- Miskulin DC, Meyer KB, Athienites NV, Martin AA, Terrin N, Marsh JV, Fink NE, Coresh J, Powe NR, Klag MJ, Levey AS. Comorbidity and Other Factors Associated with Modality Selection in Incident Dialysis Patients: The CHOICE Study. Am J Kidney Dis 39: 324-36, 2002
- Chung SH, Lindholm B, Lee HB. Is malnutrition an independent predictor of mortality in dialysis patients? Nephrol Dial Transplant 18:2134-2140, 2003
- United States Renal Data System 2009. Chapter 2: Incidence & Prevalence. Retrieved 10/07, 2020, from https://www.usrds.org/annual-data-report/current-adr/
- Chung SH, Noh H , Ha H, Lee HB. Optimal use of peritoneal dialysis in patients with diabetes. Perit Dial Int 29: Suppl 2:S132-4, 2009
- Winkelmayer WC, Glynn RJ, Levin R, Owen W Jr, Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int 60: 1547-1554, 2001
- Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG. Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14:415-424, 2003
- Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B. Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 64: 1071-1079, 2003
- DeOreo P. Hemodialysis Patient-Assessed Functional Health Status Predicts Continued Survival, Hospitalization, and Dialysis-Attendance Compliance. Am J Kidney Dis 30: 204-212, 1997
- López Revuelta K, García López FJ, de Alvaro Moreno F, Alonso J. Perceived mental health at the start of dialysis as a predictor of morbidity and mortality in patients with end-stage renal disease (CALVIDIA Study). Nephrol Dial Transplant. 19:2347-53, 2004
- Liem YS, Bosch J, Hunink M. Preference-Based Quality of Life of Patients on Renal Replacement Therapy: A systematic Review and Meta-Analysis. Value Health 11:733-41, 2008
- Kjellstrand CM, Buoncristiani U, Ting G, Traeger J, Piccoli GB, Sibai-Galland R, Young BA, Blagg CR. Short daily hemodialysis: Survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant 10:3283-3289, 2008
- Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA 11:1291-9, 2007
- Pauly RP, Gill JS, Rose CL, Asad RA, Chery A, Pierratos A, Chan CT. Survival among nocturnal home haemodialysis patients compared to kidney transplant recipients. Nephrol Dial Transplant 24: 2915-9, 2009.
- Piraino B, Minev E, Bernardini J, Bender FH. Does experience with PD matter? Perit Dial Int 29: 256-61, 2009.
- Schaubel D, Blake P, Fenton S. Effect of renal center characteristics on mortality technique failure on peritoneal dialysis. Kidney Int 60: 1517-1524, 2001
- Burkart J. The future of peritoneal dialysis in The United States: optimizing its use. Clin J Am Soc Nephrol 4: S125-S131, 2009
- Diaz Buxo JA, Crawford-Bonadio T. Major Difficulties the US Nephrologist Faces in Providing Adequate Dialysis. Blood Purif 25: 48-52, 2007
- Mehrotra R, Blake P, Berman N, et al. An Analysis of Dialysis Training in The United States and Canada. Am J Kidney Dis 40: 152-160, 2002
- Powe NR. Early referral in chronic kidney disease: An enormous opportunity for prevention. Am J Kidney Dis 41:505-507, 2003
- Diaz-Buxo JA. Early referral and selection of peritoneal dialysis as a treatment modality. Nephrol Dial Transplant 15:147-149, 2000
- Lorenzo V, Martı́n M, Rufino M, Hernández D, Torres A, Ayus JC. Predialysis nephrologic care and a functioning arteriovenous fistula at entry are associated with better survival in incident hemodialysis patients: An observational cohort study. Am J Kidney Dis 43:999-1007, 2004
- Davies S. Comparing Outcomes on Peritoneal and Hemodialysis: A Case Study in the Interpretation of Observational Studies. Saudi J Kidney Dis Transplant 18:24-30, 2007
- Foley R. Comparing the Incomparable: Hemodialysis versus Peritoneal Dialysis in Observational Studies. Perit Dial Int 24: 217-221, 2004
P/N 101291-01 Rev. 00 02/2011
