Exchange Volume and Position
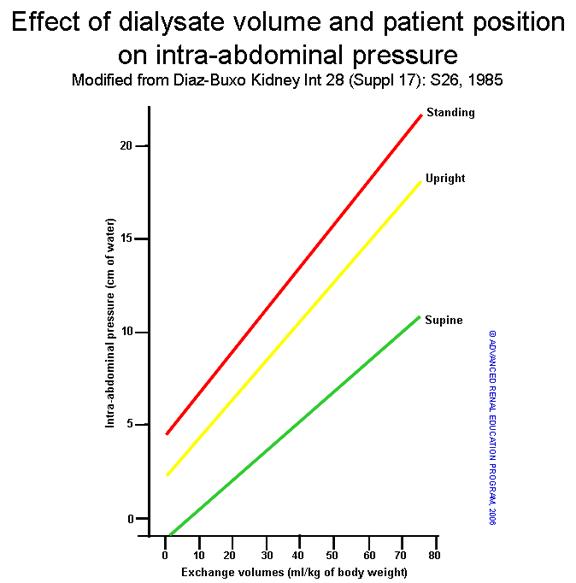
There is a direct correlation between intra-abdominal pressure (IAP) and intraperitoneal volume or exchange volume (Vip) (1) (Figure 1). The clinical determinants of IAP are ill defined, and the potential impact of elevated IAP on PD-related complications is still a matter of debate (2,3). Dejardin and coworkers showed that there was a strong linear correlation between IAP and intraperitoneal volume and a significant impact of body mass index (BMI) on IAP (4). The upper tolerated limit of IPP is 18 cmH2O in the supine position, and the normal pressure is 7 – 14 cmH2O (5). Thus, the dialysate fill volume should be low enough to be clinically well tolerated (6) and depends on IAP and peritoneal/body surface area (BSA) (7). The maximum Vip tolerated (Vmax) can be estimated using the formula:
Vmax=V+ ((18-IAP)/2)x 1000 mL
where Vmax is the maximum volume tolerated (in mL), IAP is the intraperitoneal hydrostatic pressure (in cmH2O) measured immediately before drainage of the intraperitoneal volume V (in mL). Of note, Chien et al. devised a novel formula incorporating body mass index, waist circumference, IPP and other real-time parameters from the PET for adjusting daily dwell volume to obtain greater UF (predictive accuracy 80.68%);
Daily Dwell Volume=(0.523 x waist cicrumference)+(0.852 x BMI)
The above formulas serve to provide some guidance. The resulting volume requires clinical evaluation of appropriate measures for a given patient. Increases in the prescribed volume should also be followed by measuring the Vip to confirm that pressures are adequate since clinical symptoms can be unreliable. Most adult patients generally feel comfortable with Vips of 2 to 3 L. In one study, 15 of the patients (75%) were unable to identify the exchange volumes used (9) Four of the five patients who determined the correct exchange volume for 67% to 78% of the exchanges (P < 0.04 compared with 33% expected by chance) had a body surface area greater than 1.75 m2. Of 123 exchanges, 84% were associated with no discomfort, 10% with mild discomfort, and 6% with moderate discomfort. This study showed that patients were not more likely to have discomfort with 3-L compared with lower fill volumes. In another study, 21 patients with a mean BSA of 1.66 ± 0.03 m2 were studied(10) On a scale of 0 to 10, patients’ mean discomfort scores were 2.14 ± 0.59, 3.48 ± 0.54, and 3.81 ± 0.63 (p = 0.047) at the end of the 1.5-L, 2.0-L, and 2.5-L dwells, respectively. There were no reports of cramps or shortness of breath with any fill volume. Patients were only able to correctly guess the actual fill volume in 34 of the 63 total exchanges (54.0%). In a study by Harris et al., the initial IPP increased with increasing fill volume (12.5 ± 3.7 cmH2O vs. 16.1 ± 4.2 cmH2O vs. 18.7 ± cmH2O for 2, 2.5 L, and 3L, respectively) (11) For all fill volumes, IPP had fallen by the end of the 3-hour dwell, at which time it was similar to that after an overnight 2-L exchange. The pain rating index was generally low for all exchange volumes and did not correlate with IPP. Minor degrees of discomfort were reported by 4, 2, and 1 of the patients with 3-L, 2.5-L, and 2-L exchanges, respectively. The authors suggest that despite an increased IPP, larger exchange volumes are generally well tolerated by patients, with only a minority of patients feeling mild discomfort.
Figure 1: Effect of dialysate IPP volume and patient position on IAP (1,2).
Another factor that can impact the effect of the IAP is the patient’s position. As shown in figure 1 above, the same Vip results in the lowest IAP with the patient in the supine position, intermediate in the standing position, and highest in the sitting position(12)In sitting adults with a mean BSA of 1.86 m2, the optimal IPV was shown to be ~1500 mL/m2 BSA (13), not accounting for posture. Fischbach et al. later accounted for posture and showed that an IPV of 1585 ± 235 mL/m2 BSA produced an IPP of 13.4 ± 3.1 cmH2O, well below the upper limit 18 cmH2O(14) Knowledge of these relationships could be used to provide the highest possible Vip during the night for patients in need of higher doses of therapy. Even in the absence of symptoms, the upper IPP limit should not be exceeded due to potential long-term complications such as hernia, exit site leaks, etc.
Effect of volume on clearance and ultrafiltration (UF)
Vip also has a marked effect on clearance and UF. Sarkar et al. showed that peritoneal clearances of creatinine (6.1 v 6.6 v 7.7 mL/min/1.73 m2) and urea nitrogen (7.3 v 8.6 v 9.5 mL/min) were progressively greater with increasing exchange volumes (P < 0.001)(9) These results were supported by Fukatsu et al., who also demonstrated that peritoneal urea clearance increased significantly (p < 0.001, 2.5 L vs. 2.0 L) with incremental increases in fill volume (10) With respect to UF, Durand et al. describe an inverse correlation between IAP and peritoneal UF(3) When the intraperitoneal volume is increased, the osmotic pressure of the dialysate needs to be strong enough to oppose the effects of an increase in the IAP. The relevant factor is IPP, which opposes UF as hydrostatic pressure increases. In contrast, higher fill volumes lead to slower equilibration of the glucose in the dialysate, which is the driving force for osmotic pressure resulting in ultrafiltration. Glucose concentration itself also plays a role; the higher the concentration, the longer the duration that hyperosmolarity is maintained for continued UF. At a given glucose concentration, UF first increases with volume but eventually decreases if the pressure is too high. Knowledge of the above discussed relationships could be used to provide the most appropriate Vip for individual patients.
References:
- Gotloib LA, Mines M, Garmizo L, Varka I. Hemodynamic effects of increasing intra-abdominal pressure in peritoneal dialysis. Perit Dial Bull 1:41-43, 1981
- Fischbach M, Desprez P, Donnars F, Hamel G, Geisert J. Optimization of CCPD prescription in children using peritoneal equilibration test. Adv Perit Dial. 1994;10:307-309. Available from: https://www.ncbi.nlm.nih.gov/pubmed/7999854.
- Durand PY, Balteau P, Chanliau J, Kessler M. Optimization of fill volumes in automated peritoneal dialysis. Perit Dial Int. 2000;20 Suppl 2:S83-S88. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10911649.
- Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant. 2007;22(5):1437-1444. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17308323.
- Durand PY, Chanliau J, Gambéroni J, Hestin D, Kessler M. Measurement of hydrostatic intraperitoneal pressure: a necessary routine test in peritoneal dialysis. Perit Dial Int. 1996;16 Suppl 1:S84-S87. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8728169.
- Fischbach M, Dheu C, Seugé-Dargnies L, Delobbe JF. Adequacy of peritoneal dialysis in children: consider the membrane for optimal prescription. Perit Dial Int. 2007;27 Suppl 2:S167-S170. Available from: https://www.ncbi.nlm.nih.gov/pubmed/17556298.
- Fischbach M, Dheu C, Michallat AC, Escande B, Laugel V, Barthelmebs M, Zoellner G, Schaefer F, Schmitt CP, Haraldsson B, et al. Peritoneal dialysis in children: consider the membrane for optimal prescription. Saudi J Kidney Dis Transpl. 2005;16(1):1-5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18209452.
- Chien C-C, Wang H-Y, Chien T-W, Kan W-C, Su S-B, Lin C-Y. A reference equation for objectively adjusting dwell volume to obtain more ultrafiltration in daily practice of peritoneal dialysis. Ren Fail. 2010;32(2):185-191. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20199180.
- Sarkar S, Bernardini J, Fried L, Johnston JR, Piraino B. Tolerance of large exchange volumes by peritoneal dialysis patients. Am J Kidney Dis. 1999;33(6):1136-1141. Available from: https://www.ncbi.nlm.nih.gov/pubmed/10352203.
- Fukatsu A, Komatsu Y, Senoh H, Miyai H, Tanaka Y, Oiwa T, Nakauchi M, Kasori R, Takahashi S, Kawahara H, et al. Clinical benefits and tolerability of increased fill volumes in Japanese peritoneal dialysis patients. Perit Dial Int. 2001;21(5):455-461. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11757828.
- Harris KP, Keogh AM, Alderson L. Peritoneal dialysis fill volume: can the patient tell the difference? Perit Dial Int. 2001;21 Suppl 3:S144-S147. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11887809.
- Diaz-Buxo JA. CCPD is even better than CAPD. Kidney Int Suppl. 1985;17:S26-S28. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3867797.
- Keshaviah P, Emerson PF, Vonesh EF, Brandes JC. Relationship between body size, fill volume, and mass transfer area coefficient in peritoneal dialysis. J Am Soc Nephrol. 1994;4(10):1820-1826. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8068881.
- Fischbach M, Warady BA. Peritoneal dialysis prescription in children: bedside principles for optimal practice. Pediatr Nephrol. 2009;24(9):1633-1642; quiz 1640, 1642. Available from: https://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2719743&tool=p…
P/N 102490-01 Rev. A 07-2015