Hemofiltration
Hemofiltration
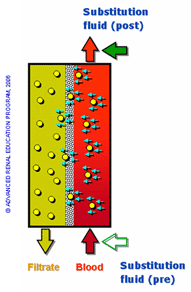
Similar to hemodialysis (HD), hemofiltration (HF) is a therapy designed to improve clearance for patients experiencing renal dysfunction. Hemofiltration is achieved by convective clearance, in which solutes are transported across a semipermeable membrane, along with movement of solvent (ultrafiltration) that occurs in response to a positive transmembrane pressure gradient (Figure 1). The clearance depends on the ultrafiltration rate and sieving characteristics of the membrane and solute and, to a lesser extent, on the molecular size of the solute. Hemofiltration involves the simultaneous removal of plasma water by ultrafiltration and replacement with a buffered electrolyte solution (replacement or reinfusion fluid)1. Usually, substitution fluid, a buffered electrolyte sterile solution similar to plasma water composition, is administered pre- or post-filter (i.e. pre-dilution mode, post-dilution mode) to account for plasma depletion that occurs during the dialysis procedure2. This is in contrast to HD, which relies on a concentration gradient (diffusion) for solute removal3. Studies comparing convective clearance and diffusive clearance have shown that middle-molecular-weight substances and large molecules are better removed by convection. Unlike HD, HF is more commonly utilized in cases of acute renal failure, in an intensive care setting, and is generally a prolonged, continuous procedure, also known as continuous renal replacement therapy, which often lasts 12 or more hours, with access usually achieved through a central venous catheter 4.
Principle:
Solute transfer across semipermeable membranes by pressure induced water flow (convection, “solute drag”)=>
-
substitution fluid (pre or post filter)
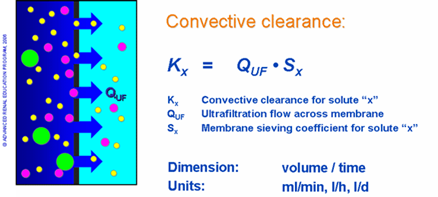
The amount of convective clearance (Kx) achieved during HF is described as the product of the respective sieving coefficient (Sx) and the ultrafiltration flow across the membrane (QUF), or total water flux, as depicted in figure 2. The rate of solute removal is therefore proportional to applied pressure, which can be adjusted to meet the needs of the clinical situation2.
Figure 2
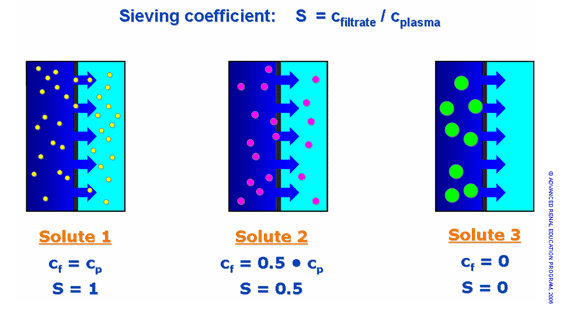
The selectivity (permeability) of the process, or the ability to distinguish different size solutes, is determined exclusively by the sieving (filtering) properties of the membrane. Thus, membrane passage of a solute is described by means of the sieving coefficient (S), which is the ratio of solute filtrate concentration (Cf) to the respective solute plasma concentration (Cp), as shown in figure 3. A sieving coefficient of one indicates unrestricted transport, while an S of zero indicates transport is completely restricted.
Figure 3
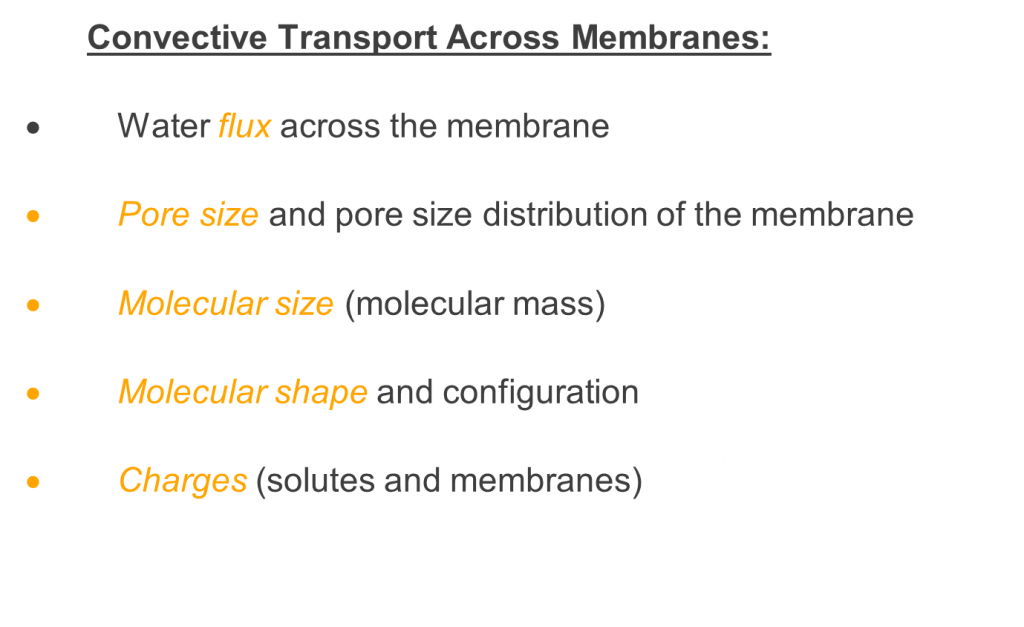
For a given membrane, each solute has a specific sieving coefficient, dependent on various factors, including primarily the solute size compared to the membrane pore size, as shown in figure 45. Other factors that determine or influence transport or convection of solutes include the molecular shape and configuration as well as possible charge effects from the solute and membrane. Solute transport may be restricted or unrestricted, based on these membrane properties 5.
Figure 4

Sieving coefficients are typically plotted versus increasing molecular mass to illustrate a sieving coefficient curve, as shown in figure 5. The graph shown below represents sieving coefficient curves for different membrane types5.
Figure 5
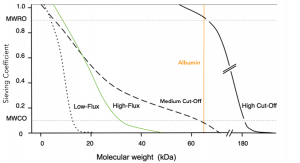
In summary, HF is based on convective transport to remove solutes from the blood of patients with renal failure. This process requires large amounts of sterile infusion fluid to compensate for fluid loss from the patient. HF is characterized by increased solute removal capabilities for higher MW solutes, but is less efficient for small MW solutes when compared to HD. This difference can be compensated by increasing exchange volumes. At present, HF is not widely employed in the routine treatment of end-stage renal disease and is generally used in the intensive care setting, in patients with acute renal failure. However, the feasibility of short daily HF as an alternative to intermittent HD is under investigation7 and could potentially lead to its increased use as a home-based renal replacement therapy moving forward.
References
- Huang Z, Letteri JJ, Ronco C, Gao D, Clark WR. CHAPTER 249 – Predilution and Postdilution Reinfusion Techniques. In: Ronco C, Bellomo R, Kellum JABT-CCN (Second E, eds. Philadelphia: W.B. Saunders; 2009:1370-1374.
- Ledebo I. Principles and practice of hemofiltration and hemodiafiltration. Artif Organs. 1998;22(1):20-25. www.ncbi.nlm.nih.gov/pubmed/9456222
- Golper TA, Fissell R, Fissell WH, Hartle PM, Sanders ML, Schulman G. Hemodialysis: core curriculum 2014. Am J kidney Dis Off J Natl Kidney Found. 2014;63(1):153-163. www.ncbi.nlm.nih.gov/pubmed/24268927
- Saunders H SD. Continuous Renal Replacement Therapy. StatPearls [Internet].
- Neri M, Villa G, Garzotto F, Bagshaw S, Bellomo R, Cerda J, Ferrari F, Guggia S, Joannidis M, Kellum J, Kim JC, Mehta RL, Ricci Z, Trevisani A, Marafon S, Clark WR, Vincent J-L, Ronco C, alliance NSI (NSI). Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care. 2016;20(1):318.
- Ronco C, La Manna G. Expanded Hemodialysis: A New Therapy for a New Class of Membranes. Contrib Nephrol. 2017;190:124-133. www.ncbi.nlm.nih.gov/pubmed/28535525
- Jaber BL, Zimmerman DL. DAILY HEMODIALYSIS—SELECTED TOPICS: Rationale and Experience with Short Daily Hemofiltration. Semin Dial. 2004;17(2):146-150.
P/N 102547-01 Rev B 05/2021