Brief History of Hemodialysis
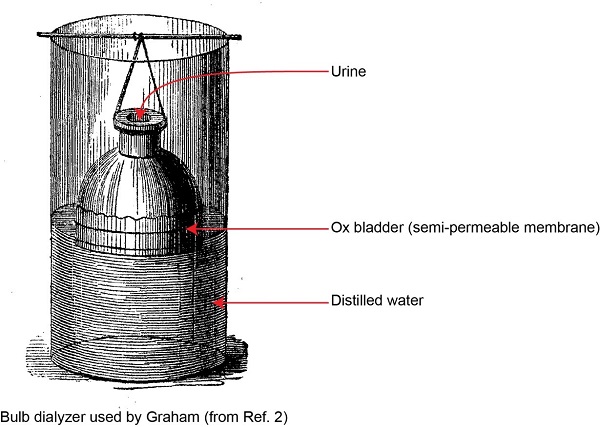
Dialysis was first described by Thomas Graham in 18541. Graham worked as a chemist in Glasgow University at around the same time as physician Richard Bright was describing the clinical features and diagnosis of renal failure in Edinburgh. Graham prepared a bell-shaped vessel shown below.
The wide open end of the bell was covered by a membrane created from an ox-bladder. He filled the bell-shaped vessel with urine and suspended it inside a larger container, filled with distilled water.
After several hours, the bell-shaped vessel was removed. The larger container was heated so that the fluid inside boiled to dryness. Graham showed that the residue in the larger container consisted mainly of sodium chloride and urea, the principal components of urine. This proved that urea had passed through the membrane. Graham termed this process dialysis and proposed, together with Richard Bright, that this would form the basis of a treatment for renal failure. They predicted that it would take around 60 years to develop the process sufficiently to be used in patients.
Aside from being the first to describe the process of separating substances with a semi-permeable membrane, Graham also was the first to separate colloids and crystalloids using a parchment membrane2. Graham realized that, for successful treatment of renal failure, toxins which accumulate in renal failure would have to be removed. It would be necessary to understand the production rate of these toxins in the patient and the rate at which they can cross the membrane. So he made many measurements of rates of transfer across the membrane for different solutes. The science of dialysis adequacy is based on a similar understanding of renal failure, uremic toxicity and membrane function.
The development of what eventually became a functional hemodialyzer was the cumulative effort of several membrane pioneers. Collodion membranes—nitrocellulose membranes derived from cotton treated with sulphuric acid, ethyl alcohol, ether and ethyl acetate—provided the first low flux dialyzers. Fick was perhaps the first to use collodion membranes to selectively separate small molecular weight solutes (MW < 5000) from blood through the process of diffusion in 18553. This was shortly followed by the preparation of collodion tubes and the manufacturing process to control pore size and water permeability4.
While significant research with artificial membranes, including dialysis of animal blood against saline solution5 and further characterization of membrane function and structure6 was conducted between 1880 and 1913, it was not until 1914 that Abel et al. developed and tested the first efficient dialysis system at Johns Hopkins University School of Medicine7. Their “vivi-diffusion” apparatus consisted of a filtering device made of cellulose trinitrate (collodion) tubes and an attached burette containing hirudin solution obtained from leech heads used as anticoagulant. That same year, Hess and McGuigan recommended high blood flows to avoid clotting or need for anticoagulation8.
The first human hemodialysis was performed in a uremic patient by Haas in 1924 at the University of Giessen in Germany9,10. He used a tubular device made of collodion immersed in dialysate solution in a glass cylinder. Haas was able to calculate that the total non-protein nitrogen removed was 2,772 g. He also showed that the presence of some uremic substances in the dialysate and that water could be removed from the blood. In 1928, he first used the anticoagulant, heparin. In 1937, the first flat hemodialysis membrane made of cellophane was produced, which is produced in similar manner to cellulose, but dissolved in alkali and carbon disulfide11. The resulting solution is then extruded through a slit and washed multiple times to obtain a transparent semipermeable material.
Willem Kolff from the Netherlands was one of the first investigators interested in the role of toxic solutes in causing the uremic syndrome. In 1940, while taking care of casualties after the German invasion of the Netherlands, his interest in acute renal failure further increased and in 1943 he introduced the rotating drum hemodialysis system using cellophane membranes and an immersion bath and the first recovery of an acute renal failure patient treated with hemodialysis was reported12,13. This was the beginning of what was to become an important clinical reality: artificial renal substitution therapy.
Significant improvements in dialyzer and equipment design occurred during the 1940’s and 50’s. Nils Alwall developed a new system with a vertical stationary drum kidney and circulating dialysate around the membrane14. He was also responsible for applying hydrostatic pressure to achieve ultrafiltration15. Kolff in turn developed the coil dialyzer using a tubular membrane wrapped around a solid core for use with a single pass dialysis fluid delivery system16. This was followed by the twin dialyzer with twin blood pathways, the first disposable hemodialyzer. In 1960, Kiil developed the plate dialyzer that could be reassembled17. The system consisted of multiple polypropylene boards supporting flat cellulosic membranes. This parallel flow kidney could be used without a blood pump due to its low resistance.
A new phase in clinical hemodialysis started with the introduction of the Quinton and Scribner AV shunt in 196018. They used silastic tubes fitted with Teflon tips into the radial artery and cephalic vein in the wrist or the posterior tibial artery and saphenous vein at the angle as an arterio-venous shunt. The two tubes ended in expanded couplings to facilitate connection. This shunt provided for the first time continuous circulation of the blood when the patient was not attached to the machine, effectively eliminating clotting and provided ready access for repeated long-term hemodialysis, opening the door to chronic renal replacement therapy.
The next significant advance in vascular access occurred later in the 1960s when Cimino and Brescia first described their native arterio-venous fistula for chronic vascular access19. These fistulas are generally created by an end-to-side vein-to-artery anastomosis. A mature native A-V fistula is by far the safest and longest lasting vascular access for hemodialysis.
The major developments over the past four decades related to improvements in membrane biocompatibility and dialyzer design, volumetric control, sophisticated monitoring systems that provide online clearances, isothermal dialysis, high flux membranes, and convective modalities such as hemofiltration and hemodiafiltration.
References
- Graham T. The Bakerian lecture: Osmotic force. Philos Trans R Soc London. 1854;144:177-228. Available from: http://rstl.royalsocietypublishing.org/content/144/177.full.pdf+html.
- Graham T. Liquid diffusion applied to analysis. Philos Trans R Soc London. 1861;151:183-224. Available from: http://rstl.royalsocietypublishing.org/content/151/183.full.pdf+html.
- Eggerth AH. The preparation and standardization of collodion membranes. J Biol Chem. 1921;48(1):203-221. Available from: http://www.jbc.org/content/48/1/203.short.
- Ferry JD. Ultrafilter Membranes and Ultrafiltration. Chem Rev. 1936;18(3):373-455. Available from: http://dx.doi.org/10.1021/cr60061a001.
- Richardson B. Practical studies in animal dialysis. Asclepiad. 1889;6:331.
- Bigelow SL, Gemberling A. Collodion membranes. J Am Chem Soc. 1907;29(11):1576-1589. Available from: http://dx.doi.org/10.1021/ja01965a005.
- Abel JJ, Rowntree LG, Turner BB. On the removal of diffusible substances from the circulating blood of living animals by dialysis. J Pharmacol Exp Ther. 1914;5(6):611-623. Available from: http://jpet.aspetjournals.org/content/5/6/611.abstract.
- Hess CL v., McGuigan H. The condition of the sugar in the blood. J Pharmacol Exp Ther. 1914;6(1):45-55. Available from: http://jpet.aspetjournals.org/content/6/1/45.short.
- Haas G. Versuche der Blutauswaschung am Lebenden mit Hilfe der Dialyse. Klin Wochenschr. 1925;4(1):13-14. Available from: http://dx.doi.org/10.1007/BF01745400.
- Benedum J. Georg Haas (1886-1971): Pionier der Hemodialse (in German). Med Hist J. 1979;14:196.
- Thalhimer W. Experimental Exchange Transfusions for Reducing Azotemia. Use of Artificial Kidney for This Purpose. Exp Biol Med . 1938;37(4):641-643. Available from: http://ebm.sagepub.com/content/37/4/641.short.
- Kolff W, Berk H. De kunstmatige nier: een dialysator met groot oppervlak (in Dutch). Ned Tijdschr Geneeskd. 1943;87:1684.
- Kolff W, Berk H, Welle NM, van der Ley A, van Dijk E, van Noordwijk J. The Artificial Kidney: a dialyser with a great area. Acta Med Scand. 1944;117(2):121-134. Available from: http://dx.doi.org/10.1111/j.0954-6820.1944.tb03951.x.
- Alwall N. On the Artificial Kidney. I Apparatus for Dialysis of the Blood in vivo. Acta Med Scand. 1947;128(4):317-325. Available from: http://dx.doi.org/10.1111/j.0954-6820.1947.tb06601.x.
- Alwall N. Therapeutic and Diagnostic Problems in Severe Renal Failure: Clinical and Experimental Studies. Thomas; 1964.
- Kolff W, Watschinger B. Further development of a coil kidney; disposable artificial kidney. J Lab Clin Med. 1956;47(6):969-977. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13319909.
- Kiil F. Development of a parallel-flow artificial kidney in plastics. Acta Chir Scand Suppl. 1960;Suppl 253:142-150. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14409028.
- Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:104-113. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13738750.
- Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275(20):1089-1092. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5923023.
P/N 102544-01 Rev B 02/2021