Novel Uremic Toxins and Atherosclerosis Risk
Focus on cardiovascular disease in the ESRD population and cross reference to the redefinition of risk factors for CV disease in the general population have generated interest in redefining the uremic status by the prevalence of markers or causative significant compounds found at increased concentration in uremia. Interest relates to compounds such as homocysteine, asymmetric dimethylargine (ADMA), p-cresol, c-reactive protein and other acute phase reactants, inflammatory cytokines such as IL-6, carbonyl radicals (aldehydes), advanced oxidation products such as AGEs and advanced oxidation protein products (AOPP). Other evidence points to a definition of a high risk dyslipidemia in ESRD characterized not only by hypertriglyceridemia and low HDL cholesterol level, but excess of small dense remnant lipoproteins, minimally modified and oxidized LDL and lipoprotein (a). A number of interested groups are examining this field of “novel” uremic toxins, seeking added rational for dialysis treatments provided at increased frequency or for time significantly greater than conventional hemodialysis.
In a collaborative study, Fagugli and colleagues examined the concentrations and dialysis kinetics of a selection of uremic toxins in 14 patients in a random two-way crossover between conventional and short daily dialysis1. Dialysis parameters, prescribed and delivered Kt/V were similar for both treatment regimens. Daily dialysis (despite equivalent weekly Kt/V) was significantly better at removing non-protein bound solutes. However, there were at best very marginal improvements in removal of protein bound solutes.
Glycation of proteins is increased in uremic and dialysis patients and advanced glycation end-products (AGEs) have been implicated in inflammation, oxidative stress and atherogenesis. Although there is some evidence that high flux dialysis can lower serum AGE levels, the effects are modest. In a recent report Floridi et al. measured a number of AGE markers (free and protein-pentosidine and two types of AGE peptides with molecular weight less than 5kD) in a study of 32 patients treated by conventional HD and converted to short daily HD2. The main findings are summarized in the figure below. Short daily dialysis resulted in a marked reduction in free pentosidine and a lesser effect on protein-bound pentosidine. Serum levels of both classes of AGE peptide also fell significantly in response to dialysis at increased frequency. These results could reflect removal of AGE precursors and AGE peptides or some other effect of daily dialysis that reduces AGE generation. The effect appears larger than seen for conventional convective therapies.
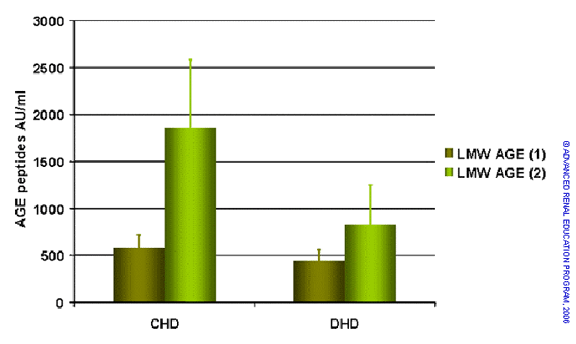
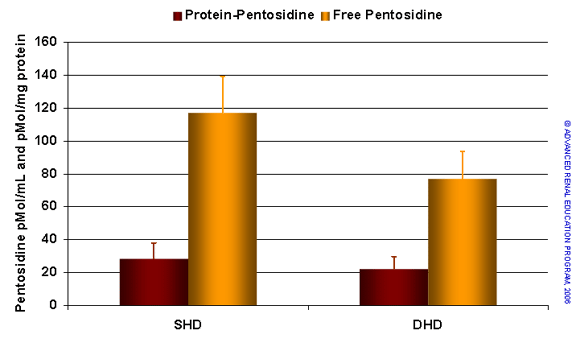 Serum levels of AGEs – free and protein-pentosidine (upper) and two classes of AGE peptide (lower) comparing conventional and short daily HD. Based on reference 2
Serum levels of AGEs – free and protein-pentosidine (upper) and two classes of AGE peptide (lower) comparing conventional and short daily HD. Based on reference 2
Homocysteine is a protein-bound solute with elevated concentration in uremic patients. Elevated homocysteine levels are associated with increased risk of cardiovascular disease. A number of authors have reported that conventional HD, whether in high- or low-flux mode, does not correct the elevated homocysteine concentration of uremia. Others have reported some improvement in hyperhomocysteinemia with use of superflux dialyzers. Floridi et al. provided preliminary evidence that short daily HD could reduce plasma total homocysteine levels as shown below3. This was, however, a cross-sectional study and must be interpreted with caution pending controlled studies. Fishbach reported a significant reduction in homocysteine in children treated with daily hemodiafiltration4.
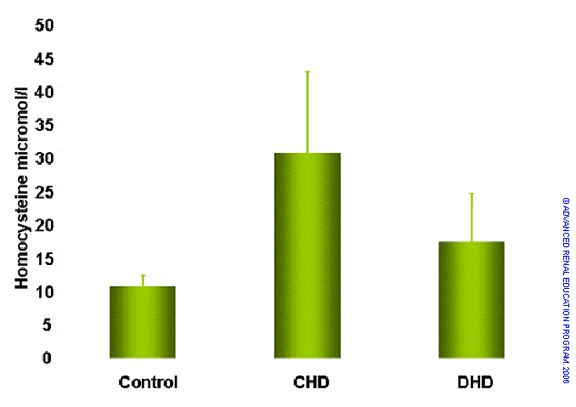 Comparison of plasma levels of total homocysteine in healthy subjects compared to patients treated by conventional and daily HD3.
Comparison of plasma levels of total homocysteine in healthy subjects compared to patients treated by conventional and daily HD3.
A number of papers describe improvement in dyslipidemia comparing high flux to low flux dialysis. Since this could not be a direct effect, it is assumed that high flux removes one or more solutes of larger size that impairs normal lipid metabolism. Bugeja et al. conducted a prospective cohort study of 11 ESRD patients before and after conversion from conventional to long nocturnal HD5. Weight, BP, hemoglobin, phosphate and albumin were measured at baseline and at 3 months after conversion to lNHD. Dialysis dose on CHD and lNHD was assessed using eKt/V. A 12-hour fasting lipid profile was obtained while on CHD and after 3 months exposure to lNHD. Three months after conversion from CHD to lNHD, a significant fall in serum triglycerides associated with a significant rise in HDL-cholesterol and HDL/total cholesterol ratio was identified as shown in the next figure. Changes in lipid profile of this magnitude would be considered a clinically significant response to any other lipid lowering therapy.
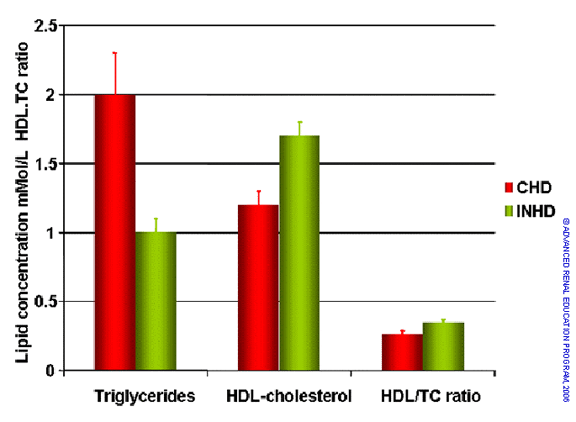 Serum levels of triglycerides, HDL-cholesterol and the ratio HDL/TC on conventional HD and three months after conversion to long nocturnal HD. Based on reference 5.
Serum levels of triglycerides, HDL-cholesterol and the ratio HDL/TC on conventional HD and three months after conversion to long nocturnal HD. Based on reference 5.
Although phosphates are small solutes, due to hydration their size in plasma water is larger than predicted by their non-hydrated molecular weight. Moreover, the predominantly intracellular location of phosphate determines kinetics that preclude adequate control of hyperphosphatemia with conventional dialysis6. On the other hand, hyperphosphatemia and a high circulating calcium-phosphate product are associated with increased risk of death and are implicated in vascular calcification, itself predictive of increased cardiovascular death, in dialyzed patients. Patients dialyzed with quotidian, long nocturnal regimes not only achieve normal serum phosphate levels, but many require the addition of phosphate salts to dialysate to prevent hypophosphatemia. By contrast, normophosphatemia without use of phosphate binders is generally not achieved with short daily regimens. A comparison of the two prescriptions was reported by Lindsay et al. 7. The trends for serum calcium-phosphate product are shown. The requirement for phosphate binders was greatly reduced in the lNHD patients and moderately reduced for patients on short daily.
Serum calcium-phosphate product in patients treated by short daily (SD) and long nocturnal (LN) dialysis compared to matched controls (C)7.
Galland reported similar findings for their patients treated by short daily dialysis (figure 18)8. There was a significant increase in phosphate intake associated with an increase in protein intake. Despite this and no increase in use of phosphate binders, serum phosphates fell significantly on short daily dialysis.
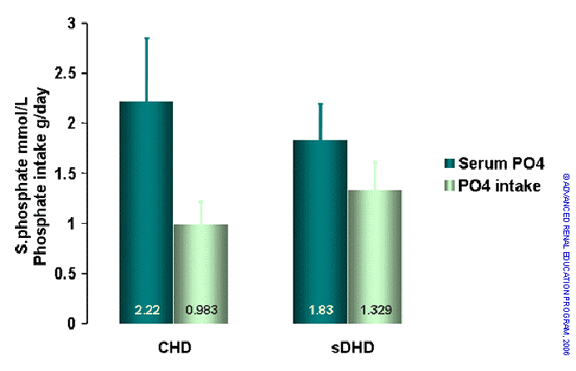 Serum phosphate and calculated dietary phosphate intake for conventional HD and short daily dialysis8.
Serum phosphate and calculated dietary phosphate intake for conventional HD and short daily dialysis8.
References:
-
Fagugli RM, De Smet R, Buoncristiani U, Lameire N, Vanholder R. Behavior of non-protein-bound and protein-bound uremic solutes during daily hemodialysis. Am J Kidney Dis 40:339-347, 2002
-
Floridi A, Antolini F, Galli F, Fagugli RM, Floridi E, Buoncristiani U. Daily haemodialysis improves indices of protein glycation. Nephrol Dial Transplant 17:871-878, 2002
-
Floridi A, Buoncristiani U, Fagugli RM, Cantelmi MG, Covarelli C. Daily hemodialysis (DHD) effectively lowers hyperhomocysteinemia in uremic patients. J Am Soc Nephrol 9:a1187,1998 (Abstract)
-
Fischbach M, Terzic J, Laugel V, Dheu C, Menouer S, Helms P, Livolsi A. Daily on-line haemodiafiltration: a pilot trial in children. Nephrol Dial Transplant 19:2360-2367, 2004
-
Bugeja AL, Chan CT. Improvement in lipid profile by nocturnal hemodialysis in patients with end-stage renal disease. ASAIO J 50:328-331, 2004
-
Leypoldt JK. Kinetics of beta-microglobulin and phosphate during hemodialysis: Effects of treatment frequency and duration. Semin Dial 18:401-408, 2005
-
Lindsay RM, Alhejaili F, Nesrallah G, Leitch R, Clement L, Heidenheim AP, Kortas C. Calcium and phosphate balance with quotidian hemodialysis. Am J Kidney Dis 42 (Suppl 1):s24-s29, 2003
-
Galland R, Traeger J, Arkouche W, Cleaud C, Delawari E, Fouque D. Short daily hemodialysis rapidly improves nutritional status in hemodialysis patients. Kidney Int 60:1555-1560, 2001
