Peritonitis in Peritoneal Dialysis
The information and reference materials contained in this document are intended solely for the general education of the reader. It is intended to provide pertinent data to assist you in forming your own conclusions and making decisions. This document should not be considered an endorsement of the information provided nor is it intended for treatment purposes and is not a substitute for professional evaluation and diagnosis. Additionally, this information is not intended to advocate any indication, dosage or other claim that is not covered, if applicable, in the FDA-approved label.
The following treatment recommendations provide a summary of best clinical practices based on the revised guidelines of the International Society of Peritoneal Dialysis (ISPD) issued in 2010 and 2016. For complete data, please refer to the original publication by Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update Perit Dial Int 30:393-423, 2010 and ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 36(5):481-508, 2016. The recommendations are applicable to adult PD patients only. This article discusses peritonitis in regards to diagnosis, treatment, subsequent infection, and Continuous Quality Improvement (CQI). Exit-site and tunnel infections are discussed in a separate series of articles on the Advanced Renal Education Program website.
Introduction to Peritonitis in Peritoneal Dialysis
Peritonitis is the inflammation of the peritoneum or peritoneal membrane. This type of inflammation typically has an infectious etiology that is mainly caused by bacteria (~80% of cases). Bacterial infections mainly come from contamination during a peritoneal dialysis (PD) session. Fungal infections are not as common as bacterial infections (only 3-6% of cases) but may occur subsequent to antibiotic use. Peritonitis is a major complication of PD due to the structural changes in the peritoneal membrane that result from PD. Approximately 4% of peritonitis episodes result in death. Since peritonitis is one of the more common complications of PD, it has caused patients to switch from this modality to hemodialysis. When a patient is diagnosed with peritonitis, the goal is to empirically treat after sampling the effluent for culture and then targeting therapy after culture results are obtained, in order to preserve the function of the peritoneal membrane as best as possible.
Diagnosis of Peritonitis in Peritoneal Dialysis
The guidelines recommend a diagnosis of peritonitis with at least two of the following:
- Clinical signs and symptoms such as abdominal pain and/or cloudy effluent
- Dialysis effluent white cell count >100 cells/µL after a dwell time of ≥2 hours and >50% of cells are polymorphonuclear
- Positive dialysis effluent culture
PD patients presenting with cloudy effluent should be presumed to have peritonitis and treated as such until a diagnosis of exclusion is reached. The PD effluent should be tested for cell count, differential, Gram stain, and culture when peritonitis is suspected.
Patients with peritonitis usually present with cloudy effluent and abdominal pain. Peritonitis should be included in the differential diagnosis even if patients present with only one of these symptoms. Cloudy effluent usually represents infectious peritonitis, but there could be differential diagnoses aside from infectious peritonitis, such as chemical peritonitis which is a possible side effect of calcium channel blockers, eosinophilia of the effluent, hemoperitoneum, malignancy, chylous effluent, or specimen taken from a dry abdomen.
Patients should be asked about any recent contamination, accidental disconnection, endoscopic or gynecologic procedure, and whether they have constipation or diarrhea. They should also be asked about their past history of peritonitis or exit-site infection (ESI).
For physical examination, patients typically have generalized abdominal tenderness with rebound. Localized pain should raise suspicion of underlying surgical pathology. Catheter tunnel and exit site should be inspected for any discharge. If there is any discharge from the exit site, it should be cultured.
Identification of Causative Organism
The ISPD guidelines recommend culturing the PD effluent with a blood-culture bottle. Protocol review of sampling and methods is recommended if more than 15% of peritonitis episodes are culture-negative. Appropriately culturing PD effluent is crucial for identifying the causative organism(s). In over 75% of cases, identification of the microbial organism can occur in less than three days. Once identified, subsequent cultures for clinical monitoring may be performed by only inoculating the effluent in blood-culture bottles. If cultures remain negative after 3 to 5 days, the effluent should have a repeat analysis for cell count, differential count, fungal, and mycobacterial culture. In addition, subculture on media with aerobic and/or microaerophilic incubation for 3 to 4 days may help identify slow-growing fastidious bacteria and yeasts that are undetectable by some automated culturing systems.
Treatment of Peritonitis as Suggested by the ISPD 2016 Guidelines
Empiric Antibiotic Selection
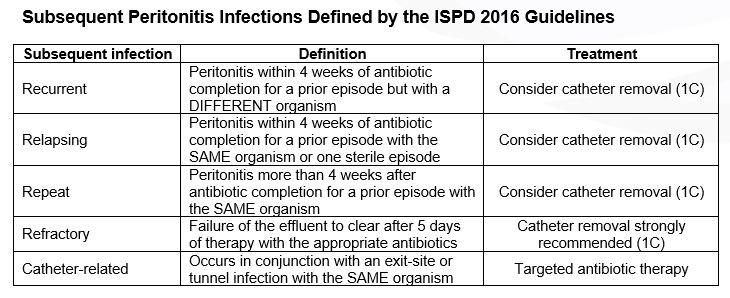
The guidelines also recommend that empiric antibiotic therapy should be initiated as soon as possible after appropriate microbiological specimens have been obtained. In addition, empiric antibiotic regimens should be center-specific and cover both Gram-positive and Gram-negative organisms. Gram-positive organisms are covered by vancomycin or a first generation cephalosporin (e.g., cefazolin), and Gram-negative organisms are covered by a third generation cephalosporin (e.g., ceftriaxone or ceftazidime) or aminoglycoside.
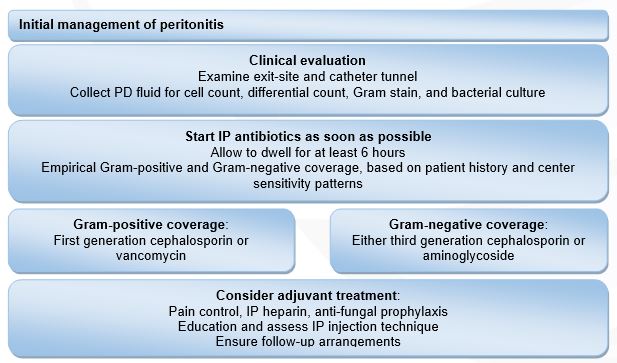
Figure 1: Initial management of peritonitis. Modified from: Li PK, Szeto CC, Piraino B, et al. ISPD Update on prevention and treatment. Perit Dial Int. 2016;36(5):481-508.
Antibiotic therapy should be adjusted to the appropriate narrow-spectrum agents once culture results and sensitivities are identified. Most PD patients with peritonitis will show considerable clinical improvement within 48 hours of initiating therapy. If no improvement occurs after 48 hours, cell counts and repeat cultures should be performed.
Treatment of Gram-positive Organisms
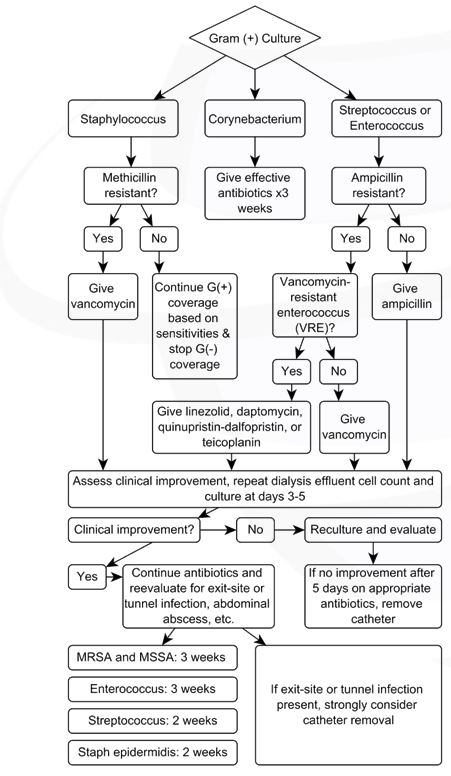
Figure 2: Gram-positive culture. Modified from: Li PK, Szeto CC, Piraino B, et al. ISPD Update on prevention and treatment. Perit Dial Int. 2016;36(5):481-508.
Treatment of Gram-negative Organisms
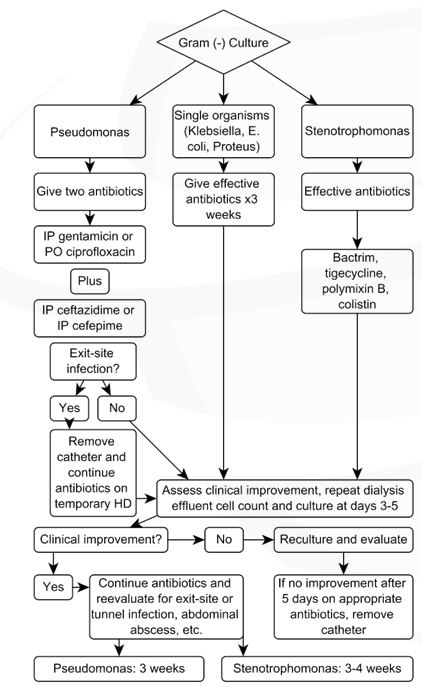
Figure 3: Gram-negative culture. Modified from: Li PK, Szeto CC, Piraino B, et al. ISPD Update on prevention and treatment. Perit Dial Int. 2016;36(5):481-508.
Treatment of Polymicrobial, Culture Negative, and Fungal Peritonitis
If the PD effluent grows multiple enteric (Gram-negative) organisms, there is a possibility of intra-abdominal pathology. Patient should be treated with metronidazole in conjunction with IP vancomycin and either IP aminoglycoside or IP ceftazidime for a minimum period of 3 weeks. Monotherapy with a carbapenem or piperacillin-tazobactam may be considered. In addition, the 2016 guidelines recommend immediate surgical evaluation when there is no prompt clinical response to antibiotics.
Polymicrobial peritonitis due to multiple Gram-positive organisms has a favorable prognosis and is most likely caused by touch contamination. The patient should be treated with effective antibiotics for 3 weeks.
Culture negative peritonitis may be due to recent antibiotic usage or technical reasons. The guidelines recommend repeat WBC count with differential if the effluent yields no growth after 3 days. If the peritonitis has not clinically improved at day 3, special culture techniques that test for mycobacteria, nocardia, legionella, filamentous fungus, or other fastidious bacteria should be considered. Initial therapy may be continued for 2 weeks if clinical improvement is noted and the effluent clears quickly. However, if improvement is not observed in 5 days, catheter removal should be strongly considered.
Fungal peritonitis is a serious complication associated with high rates of hospitalization, catheter removal, transfer to hemodialysis, and death. Catheters should be removed immediately once fungi are identified for improved outcomes and reductions in mortality. Initial antifungal therapy is a combination of amphotericin B and flucytosine and should be continued for at least 2 weeks after catheter removal. Alternative agents include fluconazole, posaconazole, voriconazole, or echinocandins.
Treatment of Mycobacterial Peritonitis
The diagnosis of tuberculous peritonitis should be considered for refractory or relapsing peritonitis with negative cultures. Special culture techniques should be considered. Treatment should be based on a 4 drug regimen: rifampicin, isoniazid, pyrazinamide, and ofloxacin, if diagnosed. Pyrazinamide and ofloxacin can be stopped after 2 months but rifampin and isoniazid should be continued for a total of 12 to 18 months. Patients often respond to therapy without catheter removal.
There is increasing data on peritonitis due to non-tuberculous mycobacteria. The treatment regimen is not well established and should follow individualized protocols based on susceptibility. Catheter removal is usually necessary.
Catheter Removal
The guideline recommended indications for catheter removal are summarized below:
- Refractory peritonitis
- Relapsing peritonitis
- Refractory exit-site and tunnel infections
- Fungal peritonitis
Catheter removal may also be considered for:
- Repeat peritonitis
- Mycobacterial peritonitis
- Multiple enteric organisms
Simultaneous re-insertion of a new catheter is not recommended for refractory and fungal peritonitis. Antibiotics should be continued for at least 2 weeks after catheter removal for refractory peritonitis.
Patients who have had catheter removal for those indications can be considered for restarting PD. The optimal duration between removal and re-insertion has not been established but studies suggest at least 2 to 3 weeks and even longer for cases of fungal peritonitis.
Drug Dosing and Stability
Drugs that can be admixed in one dialysis solution bag include aminoglycosides, vancomycin, and cephalosporins; however, chemical incompatibility exists between penicillins and aminoglycosides and therefore, should not be mixed. The use of separate syringes is necessary for admixture of antibiotics into the same bag, in conjunction with sterile technique. When dialysis solutions contain dextrose, the time of stability of added antibiotics is variable (See Table 1).
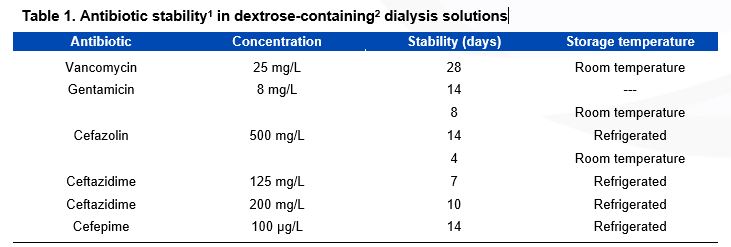
Table 1: Antibiotic Stability in Dextrose-Containing Dialysis Solutions. Modified from: Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30:393-423, 2010. It is possible that these antibiotics are stable for longer periods; more research is needed to identify the optimal stability conditions for antibiotics added to dialysis solutions; Icodextrin-containing dialysis solutions are compatible with vancomycin, cefazolin, ampicillin, cloxacillin, ceftazidime, gentamicin and amphotericin; Heparin reduces stability.
Intermittent or Continuous Dosing of Antibiotics
It is well known that the preferred method of dosing antibiotics in peritonitis is via an intraperitoneal (IP) route. Intraperitoneal dosing is favored over intravenous (IV) dosing because the local levels achieved are higher with IP administration. Additionally, the IP route is advantageous because the patient can perform it at home after adequate training. It also avoids venipuncture necessary for IV access. Optional dosing regimens of IP antibiotics include once daily (intermittent) or per each exchange (continuous). The antibiotic must dwell for a minimum of 6 hours to ensure adequate absorption.
There are few antibiotic dosing recommendations for APD patients. When APD patients are given equivalent doses as CAPD patients, significant under-dosing could occur. This can be due to rapid exchanges, where there is not enough time given for the antibiotic to be absorbed into the systemic circulation. This can be avoided by utilizing the 6 hour dwell time. Refer to the guidelines for evidence-based dosing recommendations for CAPD and APD.
The guidelines suggest that IP aminoglycosides be administered intermittently and that prolonged courses be avoided. They also suggest that IP vancomycin be administered intermittently and that serum levels be kept above 15 µg/mL. For IP cephalosporins, the guidelines suggest that they be administered continuously or intermittently.
Continuous Quality Improvement (CQI)
Peritonitis Rates
Peritoneal dialysis infection (exit-site and peritonitis) rates should be monitored and reported for every clinical dialysis program annually. The overall peritonitis rate should not exceed 0.5 episodes per year at risk. In some outstanding centers, peritonitis rates as low as 0.18 to 0.20 episodes per year have been reported. Additionally, the PD team comprised of physicians and nurses, should review the presumed etiology, causative organisms, and antibiotic sensitivity of each infection. When infection rates are increasing or undesirably high, interventions should be employed. The three main methods of reporting infections due to PD include using rates, percentages, and median rates for each program each year. The updated 2016 guidelines recommend that every program should monitor and record peritonitis incidence, the overall rate of peritonitis, rates of specific organisms, percentage of patients who are peritonitis free, and susceptibilities yearly. The guidelines suggest using a standard number of episodes per patient-year for reporting and also that absolute rates should be reported as part of a continuous quality improvement (CQI) program. The purpose of data documentation is to evaluate the program’s treatment regimen and to facilitate the best possible outcomes for patients. If the protocol is not effective in the treatment or prevention of peritonitis, it should be reassessed and changes should be implemented to achieve recommended goals.
Prevention of Peritonitis
In order to prevent peritonitis, both exit-site infections and catheter-tunnel infections must be prevented. The guidelines recommend prophylactic antibiotic therapy right before insertion of the catheter. No single catheter has been shown to have better outcomes than another in regards to peritonitis rates but ISPD does recommend disconnect systems with a “flush before fill” design with continuous ambulatory PD (CAPD). In addition, the guidelines recommend training programs for patients and that they be conducted by health care providers with experience. To prevent exit-sit infections, the guidelines recommend topical antibiotics like mupirocin or gentamicin daily. If an exit-site infection develops, treatment should be prompt and targeted to decrease the risk of peritonitis development.
Patient Education
Patients should immediately report to the PD nurse any symptoms of abdominal pain, cloudy effluent, or fever. The cloudy dialysate fluid should be drained, saved, and taken to the clinic for analysis. The patient should understand that treatment involves antibiotic therapy for 2 weeks or more. Upon completion of therapy, the patient should report any persistent cloudiness or worsening symptoms to the PD nurse. Retraining to address technique issues should also be scheduled.
Conclusions
In summary, this article discussed peritonitis in regards to diagnosis, treatment, subsequent infection, and CQI.
- The main symptoms of peritonitis include abdominal pain and cloudy effluent.
- Empiric antibiotic therapy should be started soon after samples of effluent are taken for culture; empiric therapy should consist of both Gram-positive and Gram-negative coverage.
- Upon receiving results of culture and sensitivity, empiric therapy should be tailored to the most narrow spectrum antibiotic as appropriate and should be continued between 2 and 4 weeks depending on clinical response and the specific organism (Please see the Treatment of Peritonitis section for specific durations).
- The treatment goals of peritonitis include rapid resolution of inflammation by eradicating the causative organism and preserving peritoneal membrane function.
- Monitoring peritonitis rates and evaluating outcomes of peritonitis treatment are important to ensure patients are receiving the best possible treatment.
- Employing the recommendations from the ISPD 2016 guidelines may help to reduce adverse patient outcomes and improve CQI outcomes.
P/N 101349-01 Rev A 03/2017