The Evolution of PD Catheters
Peritoneal access is an essential component of the PD system. The evolution of PD catheter designs parallels the goals for an optimal peritoneal access and the availability of appropriate materials.
The goals of peritoneal access are:
- Optimal and consistent hydraulic function
- Stable interface between catheter and body
- Minimal interference with abdominal function and clothing
- Maintenance free
- Infection free
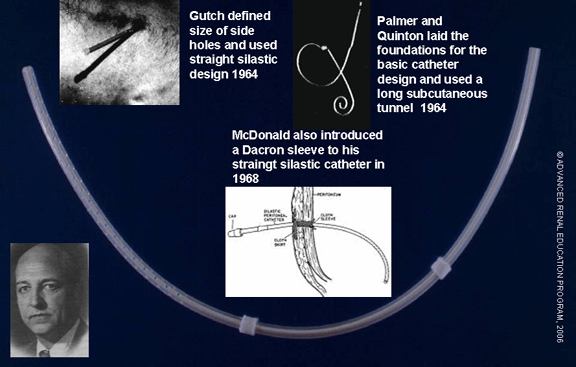
Modern peritoneal access is the result of innumerable modifications to a simple tubular structure. The process has been slow due to the many physical, biological, and immunological factors affecting its success. Many trials were carried out with conduits, to allow easy and repeated access to the peritoneum, and with catheters constructed with various materials and configurations. The balance shifted with the introduction of the Tenckhoff catheter in 1968.
Making of the Tenckhoff Catheter
In 1968, Tenckhoff and Schechter revolutionized the field of peritoneal dialysis by introducing a permanent catheter and a method of implantation that, for the first time, allowed relatively long periods of usage with a significant reduction in infections(1). The material chosen for the catheter was silicone. Silicone had shown reduced protein loss and less irritation to nearby tissue than what was seen with other catheter materials. The Palmer catheter was modified by eliminating the curled end, which they later reintroduced, shortening the length of the subcutaneous tunnel, and attaching two Dacron™ felt cuffs. One of these cuffs was placed below the skin, while the other cuff was placed inside the peritoneal cavity. The rationale for the placement of the cuffs was that enclosing the catheter tunnel from both sides offered superior protection from bacterial infection(2). Their four-part trocar allowed insertion of the catheter at the bedside with the use of local anesthesia. Tenckhoff also proposed the use of a semiarcuate tunnel with a downward exit to reduce accumulation of debris and to minimize infection.
Effective dialysate flow has been a principal concern in the design of all PD catheters. Flow (Q) is determined by the pressure gradient across the end-points of the fluid pathway (P) and the hydraulic resistance of the system (R).
Q = P/R
During inflow, the pressure gradient (P) is established by the distance between the top of the column of dialysate and the distal catheter tip. Conversely, during outflow the pressure gradient depends on the distance between the intraperitoneal space and the drain bag. As the distance between these points increases, gravity enhances flow velocity. The hydraulic resistance (R) to flow depends largely on the size of the fluid channel. Thus, the design and specifications of the peritoneal dialysis catheter, the adapter, and the tubing of the dialysis sets are major determinants of dialysate flow rate(3). The P/R ratio can be easily manipulated by changing the head height of the dialysate container, lowering the drain bag, changing the diameter of the conduits (catheter, adapter, lines) or adding a pump for infusion (positive pressure) or drainage (negative pressure).
The further evolution in PD access design responded to the need for the largest practical internal diameter, kink prevention, obstruction prevention, reduction of inflammation, biocompatible materials, and ease of implantation and removal. This led to a variety of designs with the following options:
- Curled vs. straight
- Column disc
- T-fluted
- Number of cuffs
- Cuff location
- Tunnel configuration (straight, swan-neck, pail-handle)
- Distal discs/balloons
- Materials (silicone rubber, polyurethane)
Of note, a randomized study comparing straight and coiled Tenckhoff catheters found a significantly higher frequency of flow problems due to malposition requiring catheter replacement when using the coiled catheter(4).
Photo courtesy of Dr. C. Cruz
In contrast to the great variety of designs, only two materials have been used successfully in the manufacture of PD catheters. Silicone rubber is the most frequently used plastic due to its relative biocompatibility, lack of trauma to surrounding tissues and minimal leeching of plasticizers. Polyurethane allows thinner catheter walls with larger lumina because of its higher strength. However, hydrolysis of the polyurethane surface and cracking of the material after exposure to polyethylene glycol or alcohol have been reported(5,6).
Catheter coatings with various materials have been tried with the aim of reducing the incidence of infection. Silver-coated catheters have been shown to reduce colonization in animals but not in humans(7). Antibiotic-bonded catheters failed to reduce catheter related infections, possibly due to limited choice, and short half-life, of antibiotics(8).
It is of interest that despite the many design modifications and advancements in biomaterials, even prospective randomized trials have failed to show any advantages of new catheter configurations over the traditional Tenckhoff design, with respect to mechanical and infectious complications(9,10). A prospective randomized trial published in Peritoneal Dialysis International found that catheter outcome is probably affected as much by the operator’s experience, abilities, and catheter care, as by design or material. The average catheter survival is better than 80% at one year and 70% at two years in experienced centers(11).
References
- Tenckhoff H, Schechter H. A bacteriologically safe peritoneal access device. Trans Am Soc Artif Intern Organs. 1968;14:181-187. Available from: https://www.ncbi.nlm.nih.gov/pubmed/5701529.
- Negoi D, Khanna R, Nolph K. History of Peritoneal Dialysis. In: Ing TS, Rahman M, Kjellstrand CM, eds. Dialysis History, Development and Promise. World Scientific; 2012:543-552.
- Cruz C. The peritoneal dialysis catheter. Semin Dial. 1995;8(2):103-104.
- Stegmayr BG, Wikdahl AM, Bergström M, Nilsson C, Engman U, Arnerlöv C, Petersen E. A randomized clinical trial comparing the function of straight and coiled Tenckhoff catheters for peritoneal dialysis. Perit Dial Int. 2005;25(1):85-88. Available from: https://www.ncbi.nlm.nih.gov/pubmed/15770930.
- Cruz C. Clinical experience with a new peritoneal access device. In: Ota K, Maher J, Winchester J, Hirszel P, eds. Current Concepts in Peritoneal Dialsysis. Amsterdam; 1992:164-169.
- Crabtree JH. Clinical biodurability of aliphatic polyether based polyurethanes as peritoneal dialysis catheters. ASAIO J. 2003;49(3):290-294. Available from: https://www.ncbi.nlm.nih.gov/pubmed/12790378.
- Dasgupta MK. Silver-coated catheters in peritoneal dialysis. Perit Dial Int. 1997;17 Suppl 2:S142-S145. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9163816.
- Trooskin SZ, Harvey RA, Lennard TW, Greco RS. Failure of demonstrated clinical efficacy of antibiotic-bonded continuous ambulatory peritoneal dialysis (CAPD) catheters. Perit Dial Int. 1990;10(1):57-59. Available from: https://www.ncbi.nlm.nih.gov/pubmed/2085584.
- Eklund BH, Honkanen EO, Kala AR, Kyllönen LE. Catheter configuration and outcome in patients on continuous ambulatory peritoneal dialysis: a prospective comparison of two catheters. Perit Dial Int. 1994;14(1):70-74. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8312419.
- Eklund BH, Honkanen EO, Kala AR, Kyllönen LE. Peritoneal dialysis access: prospective randomized comparison of the Swan neck and Tenckhoff catheters. Perit Dial Int. 1995;15(8):353-356. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8785234.
- Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, Strife F, Hamburger RJ. Risk factors for peritonitis in long-term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. Am J Kidney Dis. 1996;28(3):428-436. Available from: https://www.ncbi.nlm.nih.gov/pubmed/8804243.
P/N 102517-01 Rev A 07/2016